Question: good day, my question regarding the example 1.2 is, could you please in detail explain how they know where to draw the equilibrium curve? thank
good day, my question regarding the example 1.2 is, could you please in detail explain how they know where to draw the equilibrium curve? thank you kindly somewhere i missed something and i am never sure how they determine what the curve should look like I assume they dont just draw in a random curve for this but get values for it. If i insert value into the equation like (0.2;y) i dont seem to get a value on that curve, so basicly can you please in detail explain how the curve is achieve and the values for the curve. thank you kindly
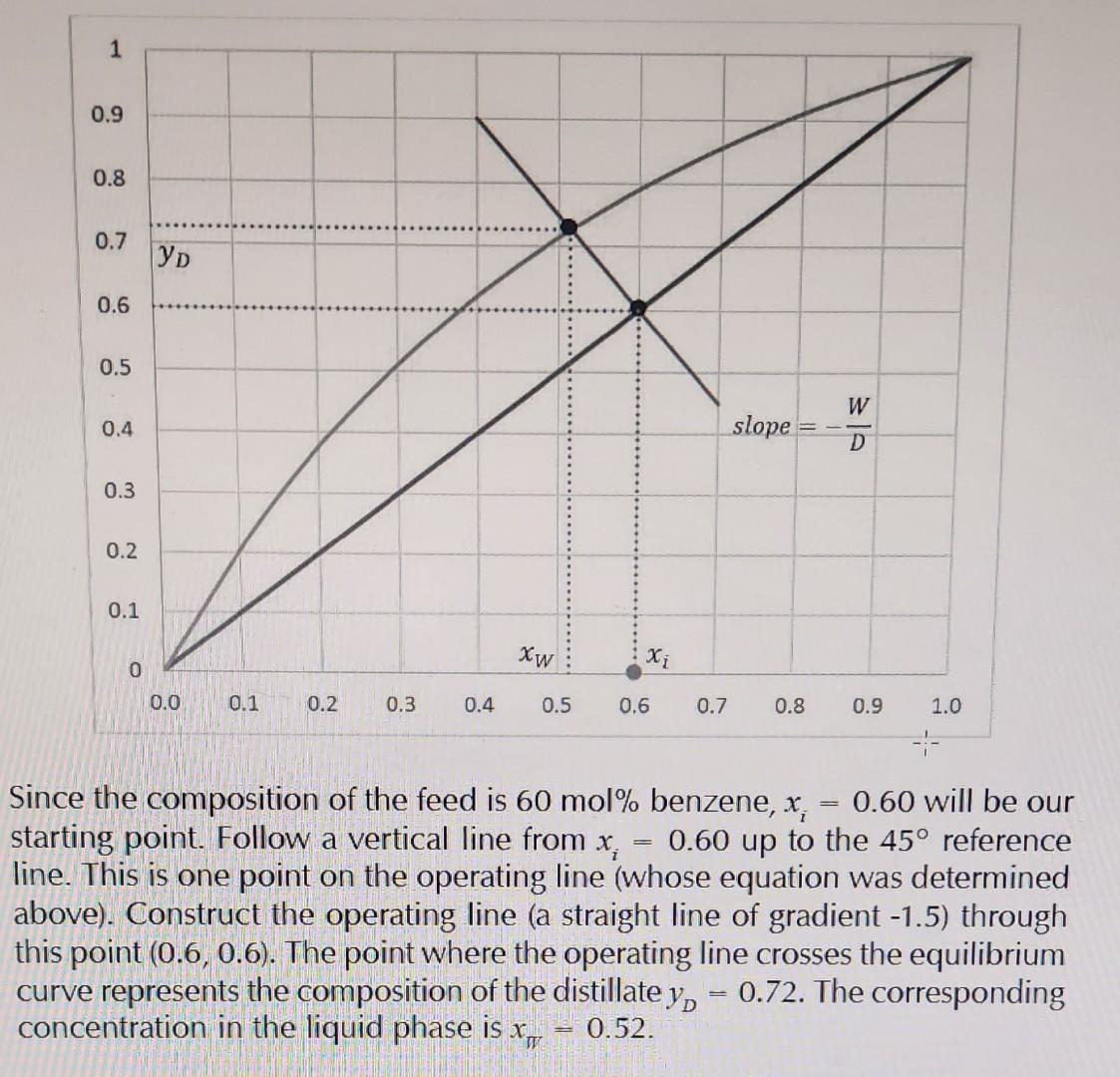
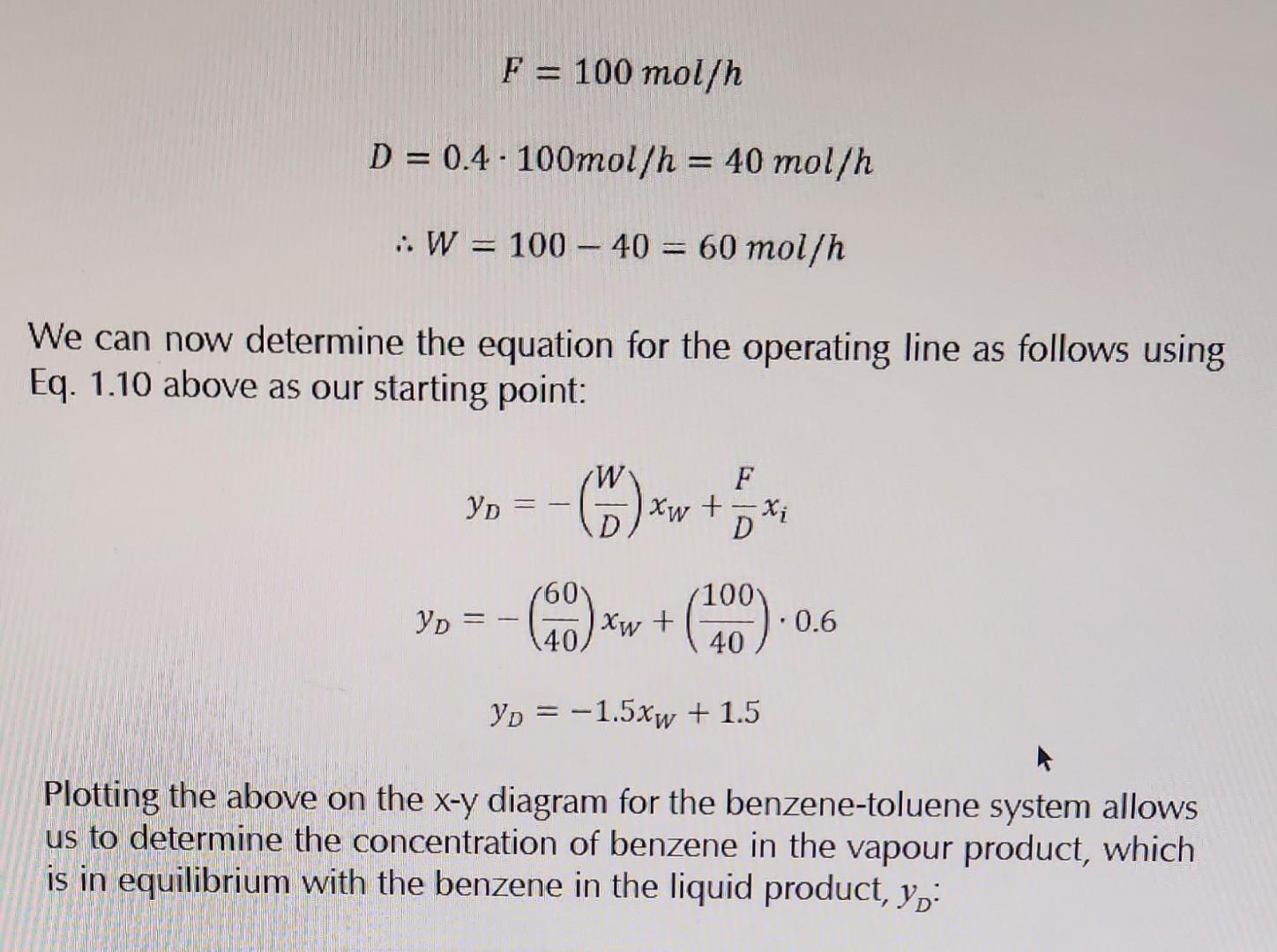

Since the composition of the feed is 60mol% benzene, xi=0.60 will be our starting point. Follow a vertical line from xi=0.60 up to the 45 reference line. This is one point on the operating line (whose equation was determined above). Construct the operating line (a straight line of gradient -1.5) through this point (0.6,0.6). The point where the operating line crosses the equilibrium curve represents the composition of the distillate yD=0.72. The corresponding concentration in the liquid phase is xm=0.52. F=100mol/hD=0.4100mol/h=40mol/hW=10040=60mol/h We can now determine the equation for the operating line as follows using Eq. 1.10 above as our starting point: yD=(DW)xW+DFxiyD=(4060)xW+(40100)0.6yD=1.5xW+1.5 Plotting the above on the xy diagram for the benzene-toluene system allows us to determine the concentration of benzene in the vapour product, which is in equilibrium with the benzene in the liquid product, yD : Consider a mixture of benzene and toluene which is to undergo equilibrium separation in a flash drum. The feed composition consists of 60mol% benzene as the more volatile component. Forty mol\% of the feed is vaporised. Determine the mole fraction of benzene in the distillate product (vapour phase). Solution Since the feed is composed of 60mol% Benzene, xi=0.6. We know that 40mol% of the feed is vaporised. Let us assume that the total feed flow rate is 100mol/h. Thus Since the composition of the feed is 60mol% benzene, xi=0.60 will be our starting point. Follow a vertical line from xi=0.60 up to the 45 reference line. This is one point on the operating line (whose equation was determined above). Construct the operating line (a straight line of gradient -1.5) through this point (0.6,0.6). The point where the operating line crosses the equilibrium curve represents the composition of the distillate yD=0.72. The corresponding concentration in the liquid phase is xm=0.52. F=100mol/hD=0.4100mol/h=40mol/hW=10040=60mol/h We can now determine the equation for the operating line as follows using Eq. 1.10 above as our starting point: yD=(DW)xW+DFxiyD=(4060)xW+(40100)0.6yD=1.5xW+1.5 Plotting the above on the xy diagram for the benzene-toluene system allows us to determine the concentration of benzene in the vapour product, which is in equilibrium with the benzene in the liquid product, yD : Consider a mixture of benzene and toluene which is to undergo equilibrium separation in a flash drum. The feed composition consists of 60mol% benzene as the more volatile component. Forty mol\% of the feed is vaporised. Determine the mole fraction of benzene in the distillate product (vapour phase). Solution Since the feed is composed of 60mol% Benzene, xi=0.6. We know that 40mol% of the feed is vaporised. Let us assume that the total feed flow rate is 100mol/h. Thus
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


