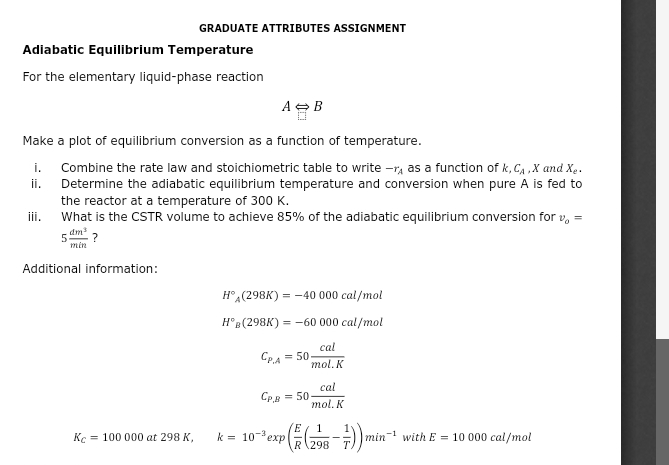
Question: GRADUATE ATTRIBUTES ASSIGNMENT Adiabatic Equilibrium Temperature For the elementary liquid - phase reaction A > B Make a plot of equilibrium conversion as a function
GRADUATE ATTRIBUTES ASSIGNMENT
Adiabatic Equilibrium Temperature
For the elementary liquidphase reaction
Make a plot of equilibrium conversion as a function of temperature.
i Combine the rate law and stoichiometric table to write as a function of and
ii Determine the adiabatic equilibrium temperature and conversion when pure is fed to the reactor at a temperature of
iii. What is the CSTR volume to achieve of the adiabatic equilibrium conversion for
Additional information:
exp with
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


