Question: HCL was used in excess making magnesium the limiting reactant. the mol of H2 calculated fot the post-lab is the theoretical yield of H2. calculate
HCL was used in excess making magnesium the limiting reactant. the mol of H2 calculated fot the post-lab is the theoretical yield of H2. calculate the actual yield of H2 using the ideal gas law and the volume pressure, and tempature data from the exercise. then calculate the percent yield. uae trials 1 and 2 for data. 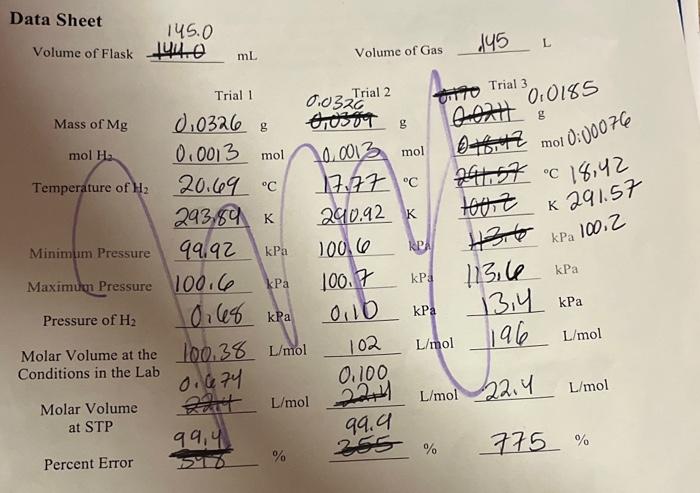

Data Sheet Volume of Flask 144.0 mL Volume of Gas .145L Percent Error 5%899,4%%
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


