Question: 1. In this exercise, HCl was used in excess, making magnesium the limiting reactant. The mol of H2 calculated for the post-lab is the theoretical

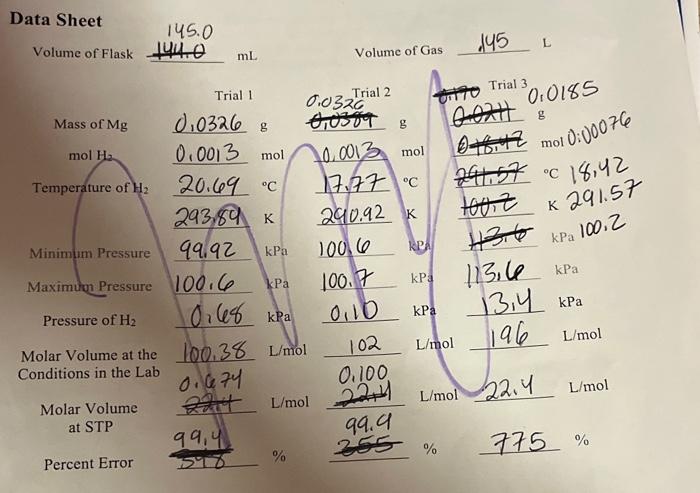
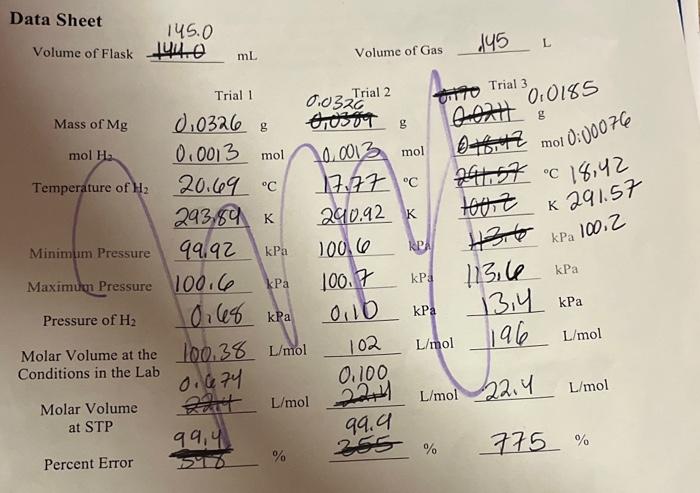
1. In this exercise, HCl was used in excess, making magnesium the limiting reactant. The mol of H2 calculated for the post-lab is the theoretical yield of H2. Choose two of your trials and calculate the actual yield of H2 using the ideal gas law and the volume, pressure, and temperature data from the exercise. Then calculate the percent yield. Trial Trial 2. Consider your results, percent errors, and percent yields from question 1 . Were you able to confirm that the molar volume of a gas at STP is 22.4L/mol ? Why or why not? Include at least one source of error as part of your answer. Data Sheet Volume of Flask 145.0144.0 mL Volume of Gas 145 Trial 3 . 0,0185 mol 0:00076 C18,42 291.57 Percent Error 59899,4%255%775% Data Sheet Volume of Flask 144.0 mL Volume of Gas .145L Percent Error 5%899,4%% 1. In this exercise, HCl was used in excess, making magnesium the limiting reactant. The mol of H2 calculated for the post-lab is the theoretical yield of H2. Choose two of your trials and calculate the actual yield of H2 using the ideal gas law and the volume, pressure, and temperature data from the exercise. Then calculate the percent yield. Trial Trial 2. Consider your results, percent errors, and percent yields from question 1 . Were you able to confirm that the molar volume of a gas at STP is 22.4L/mol ? Why or why not? Include at least one source of error as part of your answer. Data Sheet Volume of Flask 145.0144.0 mL Volume of Gas 145 Trial 3 . 0,0185 mol 0:00076 C18,42 291.57 Percent Error 59899,4%255%775% Data Sheet Volume of Flask 144.0 mL Volume of Gas .145L Percent Error 5%899,4%%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


