Question: HCN is a monoprotic weak acid with a Ka value of 4.901010. Calculate the pH of a 8.50106M solution of this acid ignoring the effects
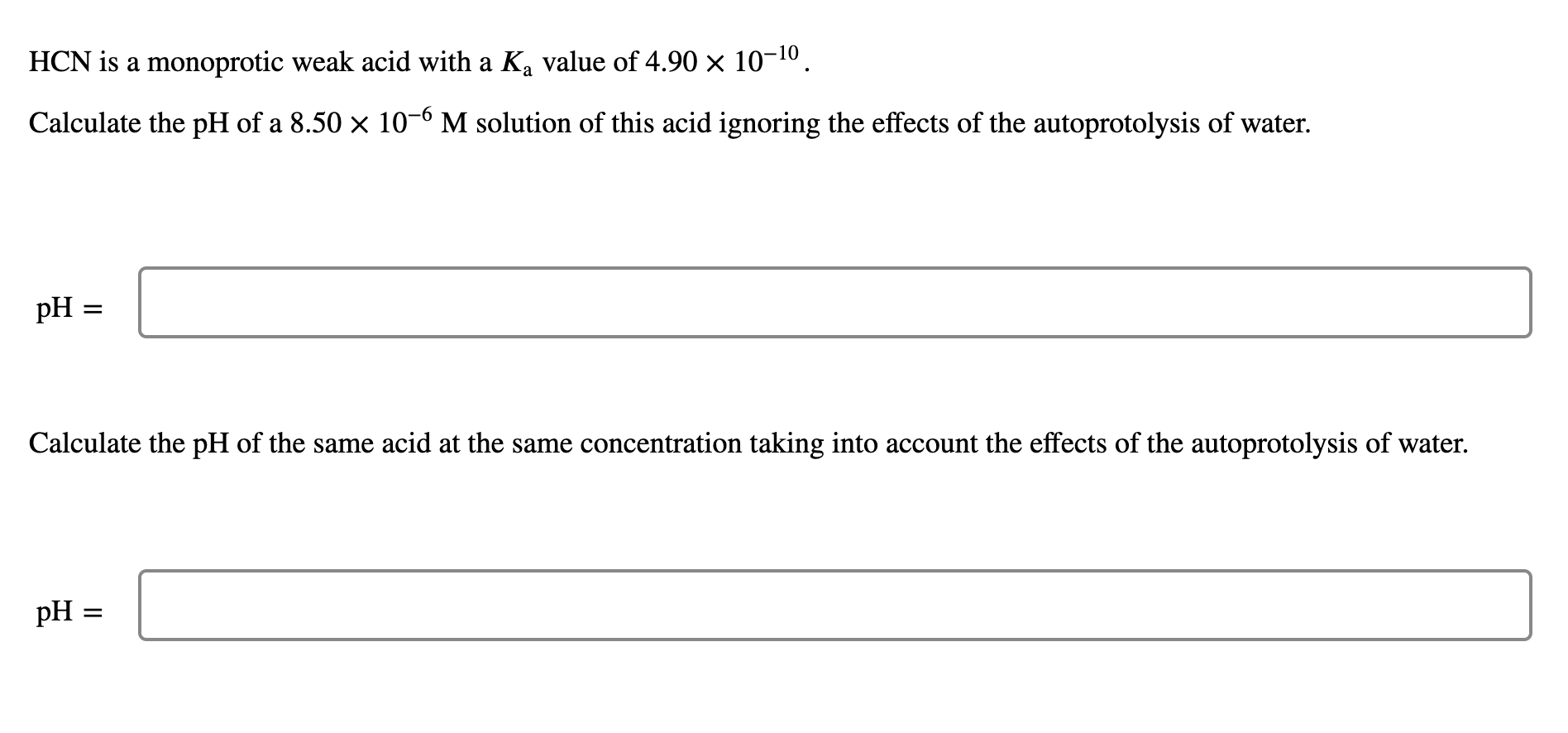
HCN is a monoprotic weak acid with a Ka value of 4.901010. Calculate the pH of a 8.50106M solution of this acid ignoring the effects of the autoprotolysis of water. pH Calculate the pH of the same acid at the same concentration taking into account the effects of the autoprotolysis of water
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


