Question: he formula for nitrogen dioxide is NO2. a. How many grams of nitrogen are present in 4.86 moles of nitrogen dioxide? grams b. How many

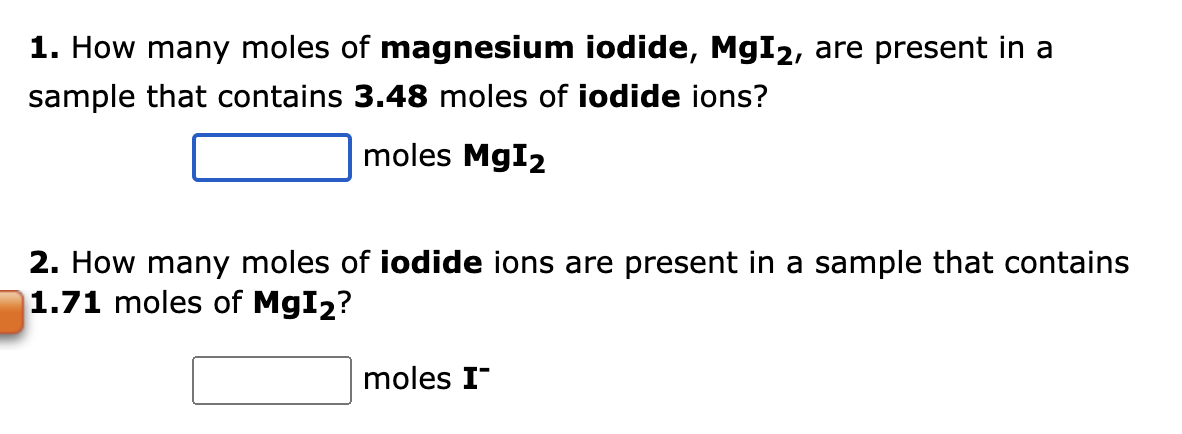
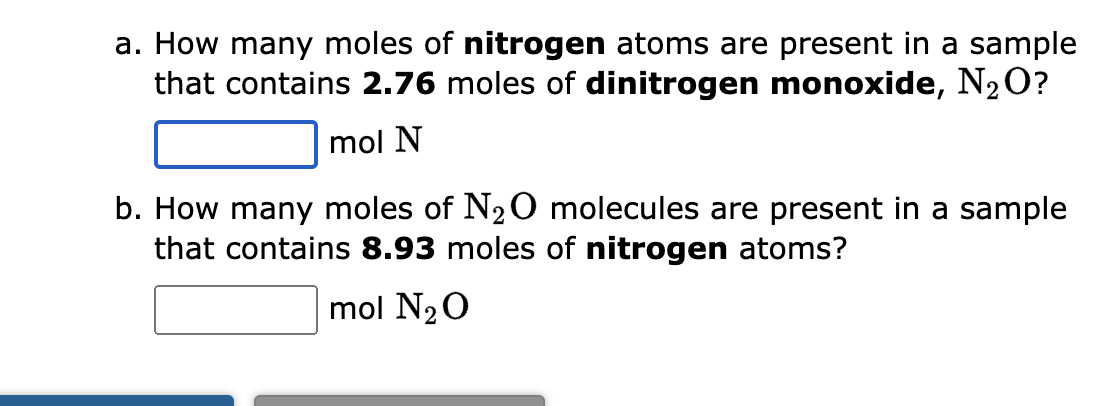
he formula for nitrogen dioxide is NO2. a. How many grams of nitrogen are present in 4.86 moles of nitrogen dioxide? grams b. How many moles of oxygen are present in 3.37 grams of nitrogen dioxide? moles 1. How many moles of magnesium iodide, MgI2, are present in a sample that contains 3.48 moles of iodide ions? molesMgI2 2. How many moles of iodide ions are present in a sample that contains 1.71 moles of MgI2 ? moles I a. How many moles of nitrogen atoms are present in a sample that contains 2.76 moles of dinitrogen monoxide, N2O ? molN b. How many moles of N2O molecules are present in a sample that contains 8.93 moles of nitrogen atoms? molN2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


