Question: Use the Rererences to access important values if needed for this qu Calculate the percentage composition for SO3. Mass percentage of sulfur = Mass percentage
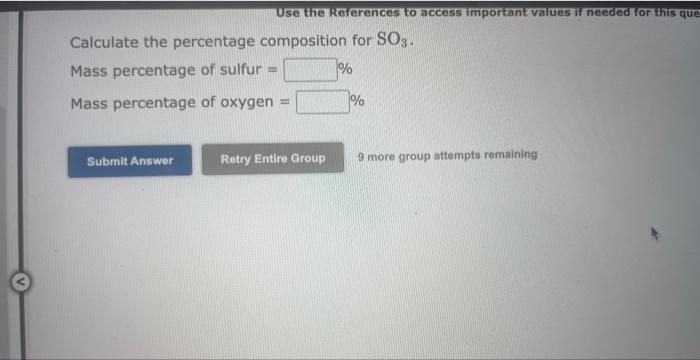
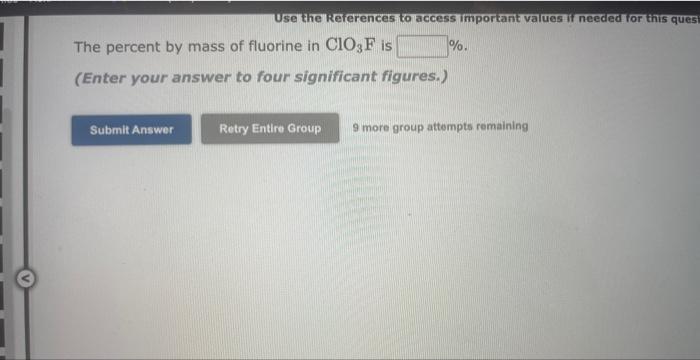
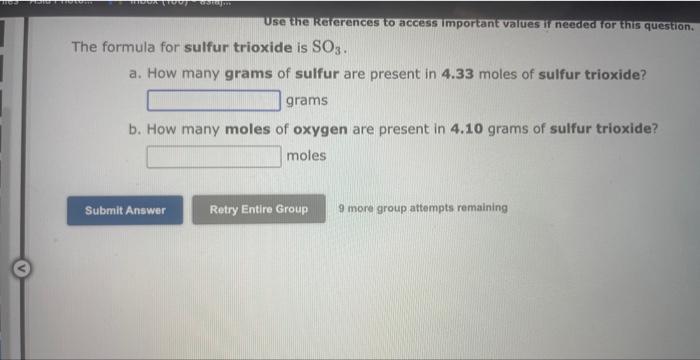
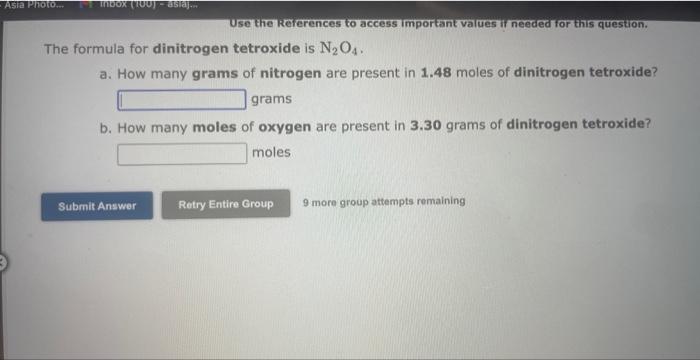
Use the Rererences to access important values if needed for this qu Calculate the percentage composition for SO3. Mass percentage of sulfur = Mass percentage of oxygen = 9 more group attempts remaining Use the kererences to access imporkant values if needed or this ques The percent by mass of fluorine in ClO3F is (Enter your answer to four significant figures.) 9 more group attempts remaining The formula for sulfur trioxide is SO3. a. How many grams of sulfur are present in 4.33 moles of sulfur trioxide? grams b. How many moles of oxygen are present in 4.10 grams of sulfur trioxide? moles 9 more group attempts remaining Use the References to access important values if needed for this question. The formula for dinitrogen tetroxide is N2O4. a. How many grams of nitrogen are present in 1.48 moles of dinitrogen tetroxide? grams b. How many moles of oxygen are present in 3.30grams of dinitrogen tetroxide? moles 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


