Question: Complete the Kal expression for H2CO3 in an aqueous solution. Kal = 4.45 10-7 = Answer Bank [H3O+] [H2CO3] [CO] [H3O+] [HCO3]
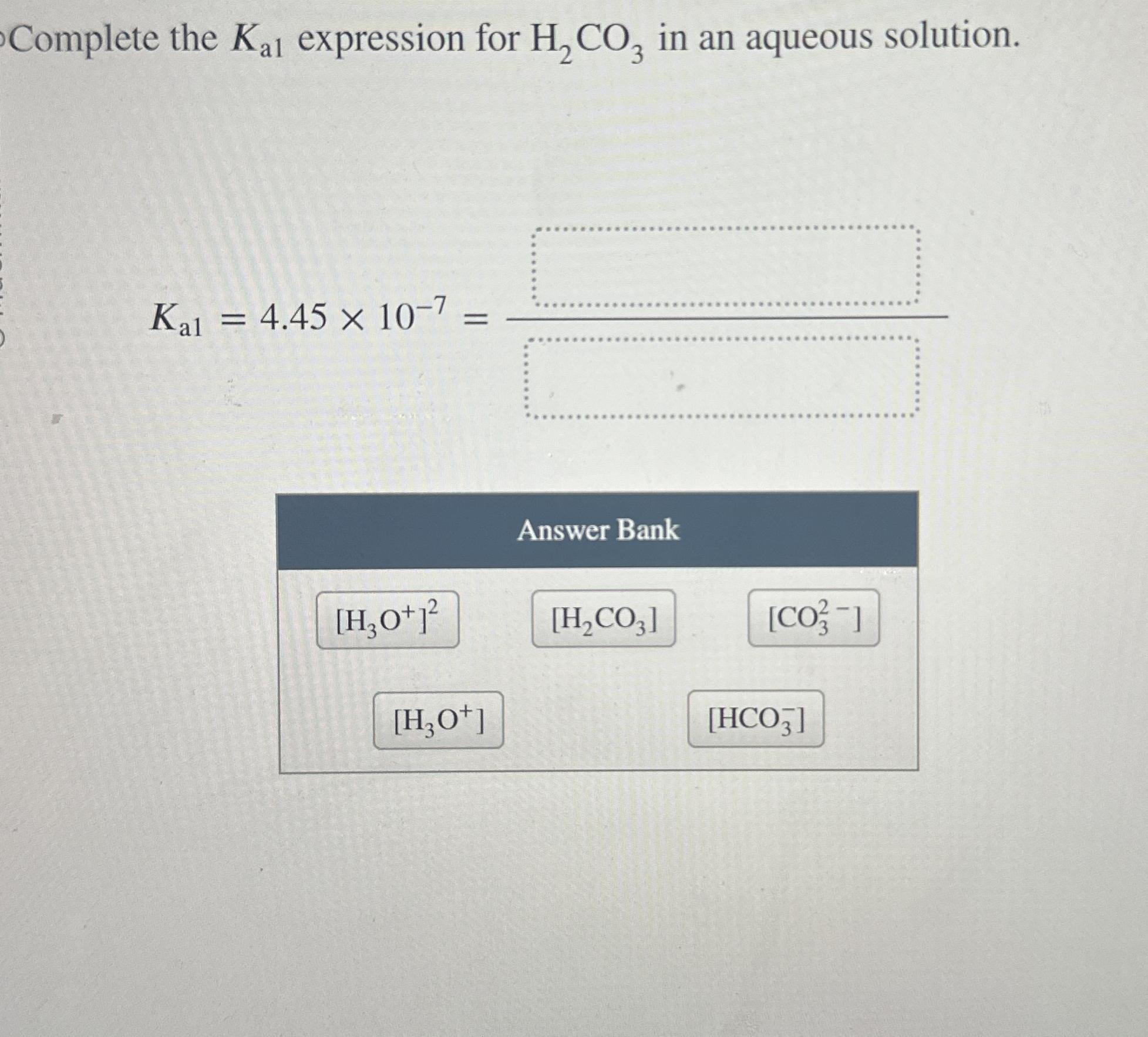
Complete the Kal expression for H2CO3 in an aqueous solution. Kal = 4.45 10-7 = Answer Bank [H3O+] [H2CO3] [CO] [H3O+] [HCO3]
Step by Step Solution
There are 3 Steps involved in it
For carbonic acid HCO the first acid dissociation is H 2 C O 3 H 2 O H C O 3 H 3 O mathrmH2CO3 ... View full answer

Get step-by-step solutions from verified subject matter experts


