Question: Hello, Can someone explain why b is the correct answer and why c and d are incorrect (in depth explanations)? thank you! The diagram above
Hello,
Can someone explain why b is the correct answer and why c and d are incorrect (in depth explanations)?
thank you!
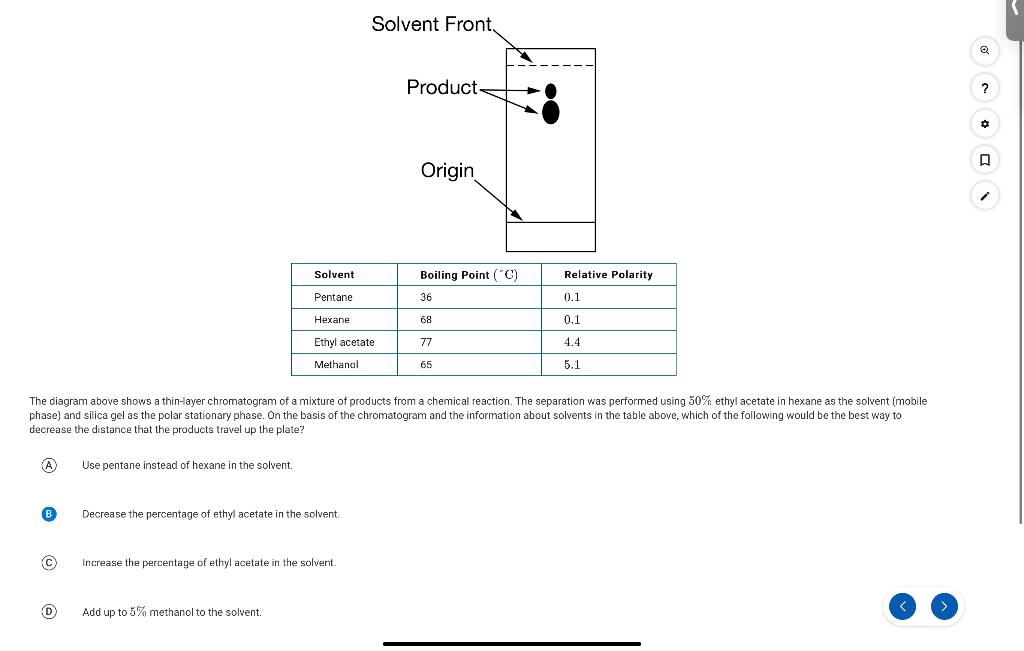
The diagram above shows a thin-layer chromatogram of a mixture of products from a chemical reaction. The separation was performed using 50% ethyl acetate in hexane as the solvent (mobile phase) and silica gel as the polar stationary phase. On the basis of the chromatogram and the information about solvents in the table above, which of the following would be the best way to decrease the distance that the products travel up the plate? Use pentane instead of hexane in the solvent. Decrease the percentage of ethyl acetate in the solvent. Increase the percentage of ethyl acetate in the solvent. Add up to 5% methanol to the solvent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


