Question: Hello can someone please help me answer this question thank you!! 8. Below you will find a table with titration data of a sample from

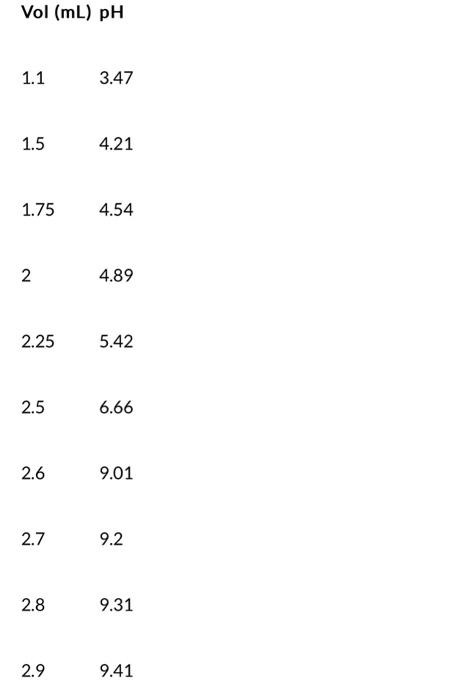
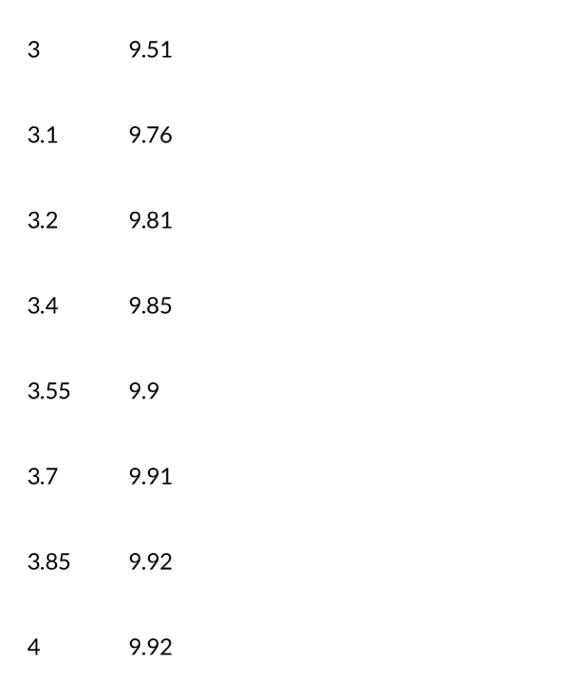
8. Below you will find a table with titration data of a sample from a batch of Kombucha that is being titrated with a standardized NaOH solution of 0.01000M. From this data below, determine the volume of the end point using a first derivative plot Notice that with this data set, the volumes being read are from a buret and the initial volume shown here is the starting point. Vol(mL)pH 1.13.47 1.54.21 1.754.54 24.89 2.255.42 2.56.66 2.69.01 2.79.2 2.89.31 2.99.41 39.51 3.19.76 3.29.81 3.49.85 3.559.9 3.79.91 3.859.92 49.92
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


