Question: Hello, could you help me with finding the normal boiling point of this liquid. (The second part of the question) I can't seem to get
Hello, could you help me with finding the normal boiling point of this liquid. (The second part of the question) I can't seem to get it correct. Thank you!
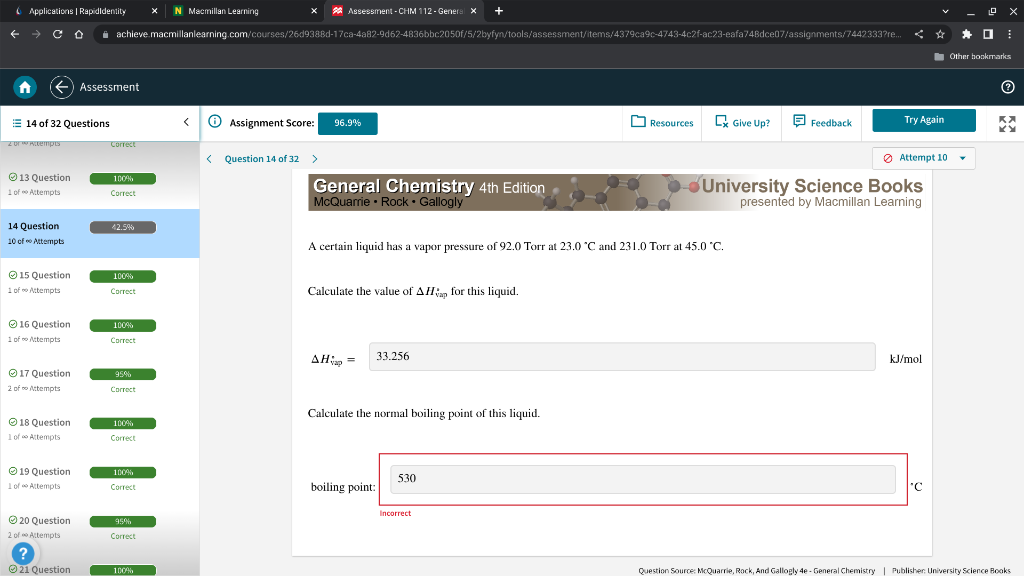
A certain liquid has a vapor pressure of 92.0 Torr at 23.0C and 231.0 Torr at 45.0C. Calculate the value of Hvap for this liquid. Hvap= kJ/mol Calculate the normal boiling point of this liquid. boiling poin 'C A certain liquid has a vapor pressure of 92.0 Torr at 23.0C and 231.0 Torr at 45.0C. Calculate the value of Hvap for this liquid. Hvap= kJ/mol Calculate the normal boiling point of this liquid. boiling poin 'C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


