Question: Hello! Could you please solve this exercise and write a python code for it, please? Thanks in advance. Exercise : The rate of the gas-phase
Hello! Could you please solve this exercise and write a python code for it, please? Thanks in advance.
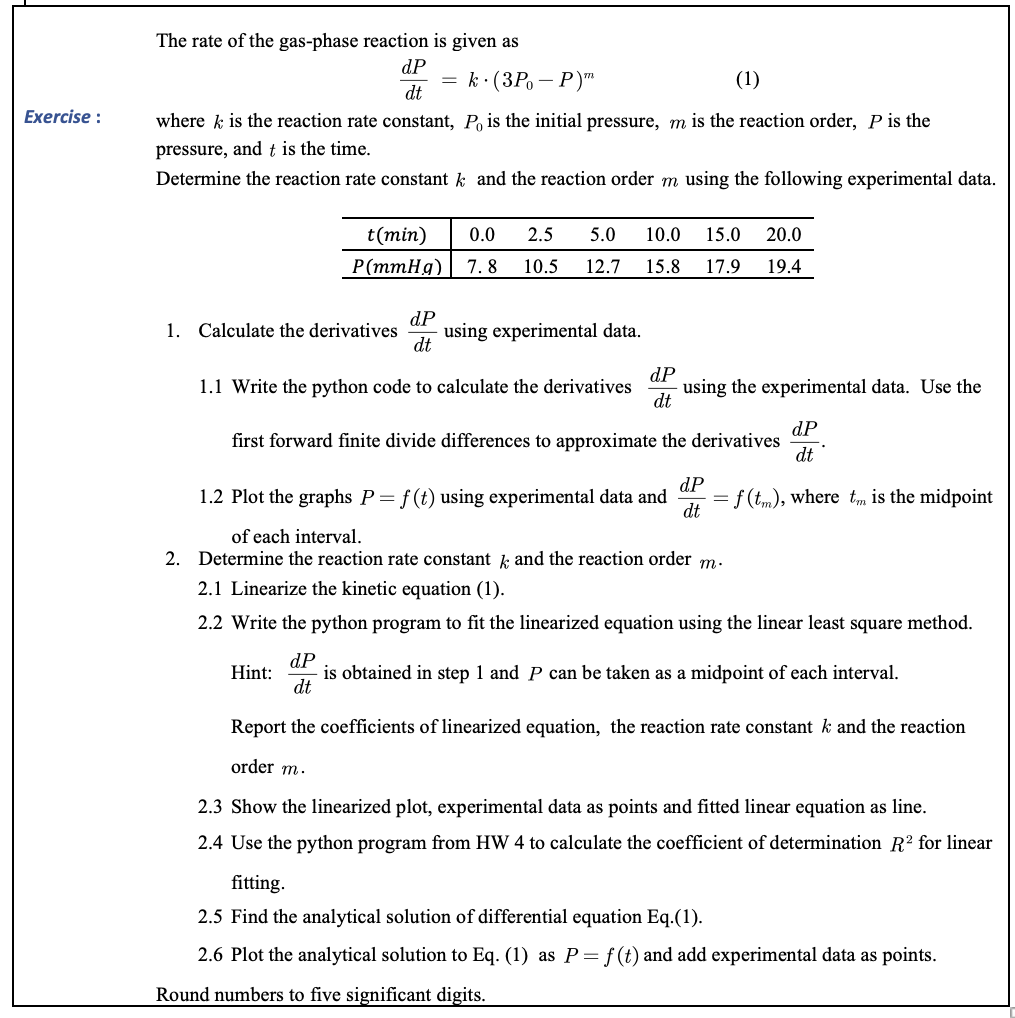
Exercise : The rate of the gas-phase reaction is given as dP = k(3P. - P)" (1) dt where k is the reaction rate constant, Po is the initial pressure, m is the reaction order, P is the pressure, and t is the time. Determine the reaction rate constant k and the reaction order m using the following experimental data. 0.0 2.5 5.0 10.0 15.0 20.0 t(min) P(mmHg) 7.8 10.5 12.7 15.8 17.9 19.4 dP 1. Calculate the derivatives using experimental data. dt dP 1.1 Write the python code to calculate the derivatives using the experimental data. Use the dt dP first forward finite divide differences to approximate the derivatives dt dP 1.2 Plot the graphs P=f(t) using experimental data and = f(tm), where tm is the midpoint dt of each interval. 2. Determine the reaction rate constant k and the reaction order m. 2.1 Linearize the kinetic equation (1). 2.2 Write the python program to fit the linearized equation using the linear least square method. dP Hint: is obtained in step 1 and P can be taken as a midpoint of each interval. dt Report the coefficients of linearized equation, the reaction rate constant k and the reaction order m. 2.3 Show the linearized plot, experimental data as points and fitted linear equation as line. 2.4 Use the python program from HW 4 to calculate the coefficient of determination R for linear fitting. 2.5 Find the analytical solution of differential equation Eq.(1). 2.6 Plot the analytical solution to Eq. (1) as P= f(t) and add experimental data as points. Round numbers to five significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


