Question: hello, I am doing a lab and need help finding Kf using my data 1st trial 6.001g naphthalene 2nd trial 6.001g naphthalene +1.019g Octadecanol Octadecanol
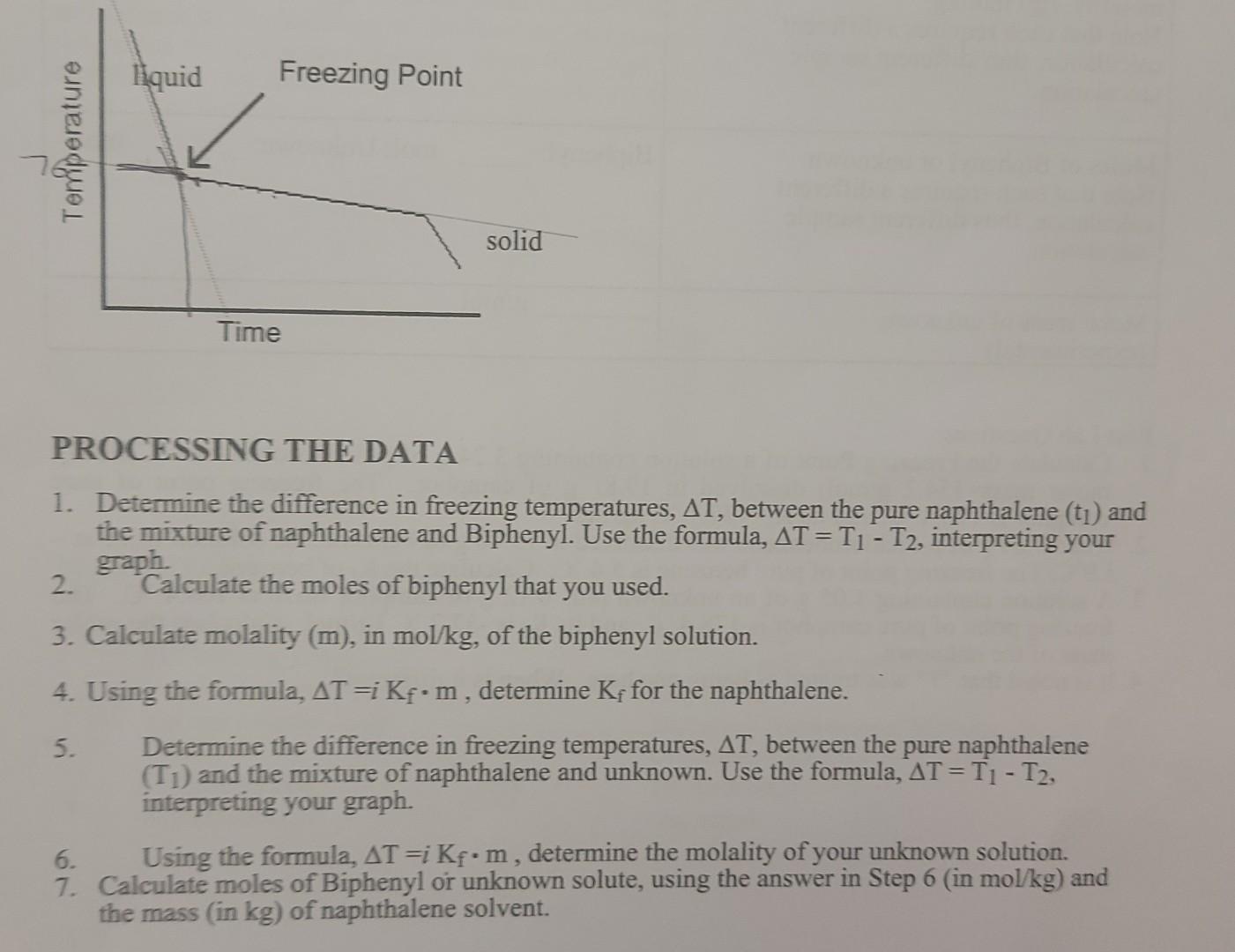
hello, I am doing a lab and need help finding Kf using my data 1st trial 6.001g naphthalene
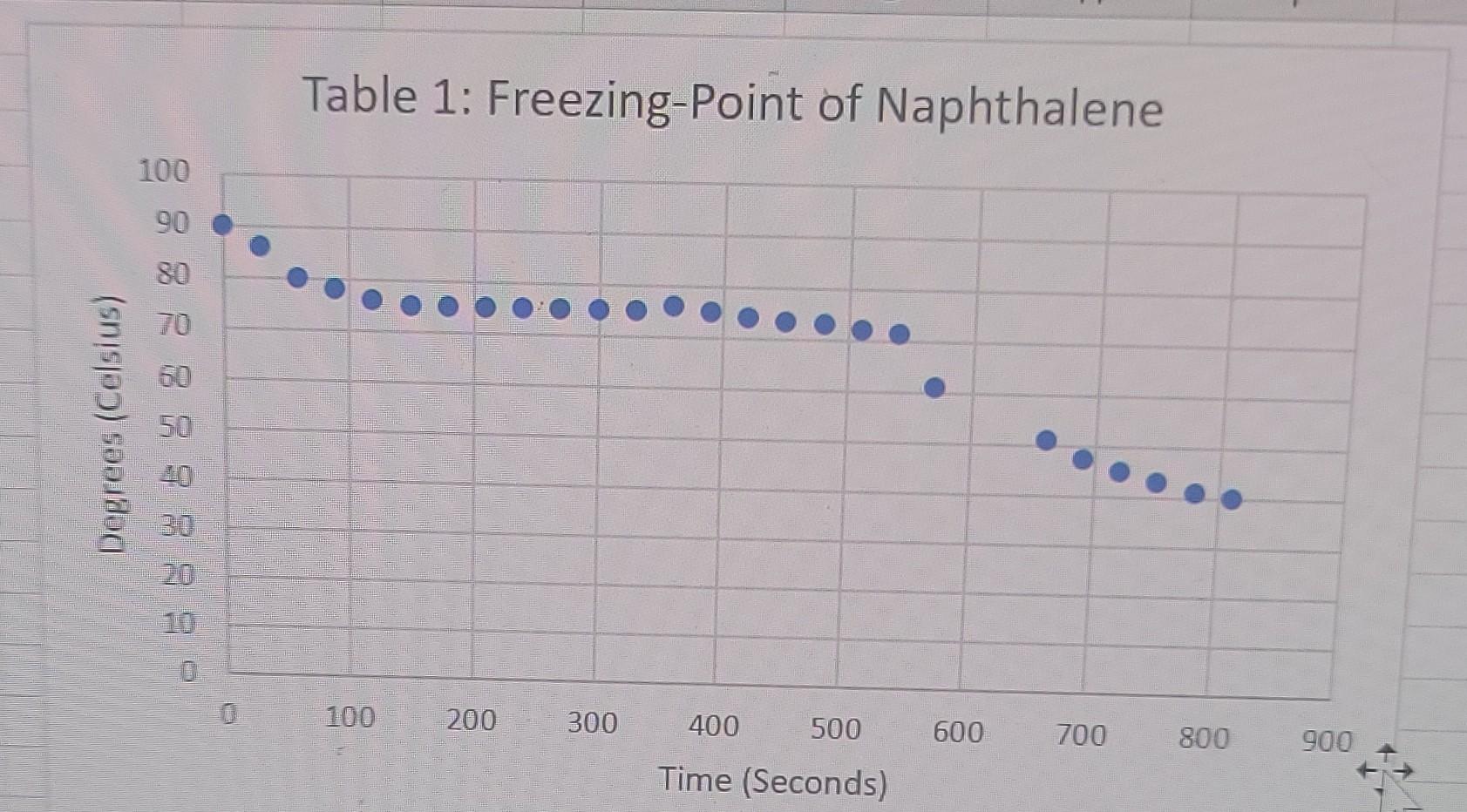
2nd trial 6.001g naphthalene +1.019g Octadecanol Octadecanol is 270.48g/mol
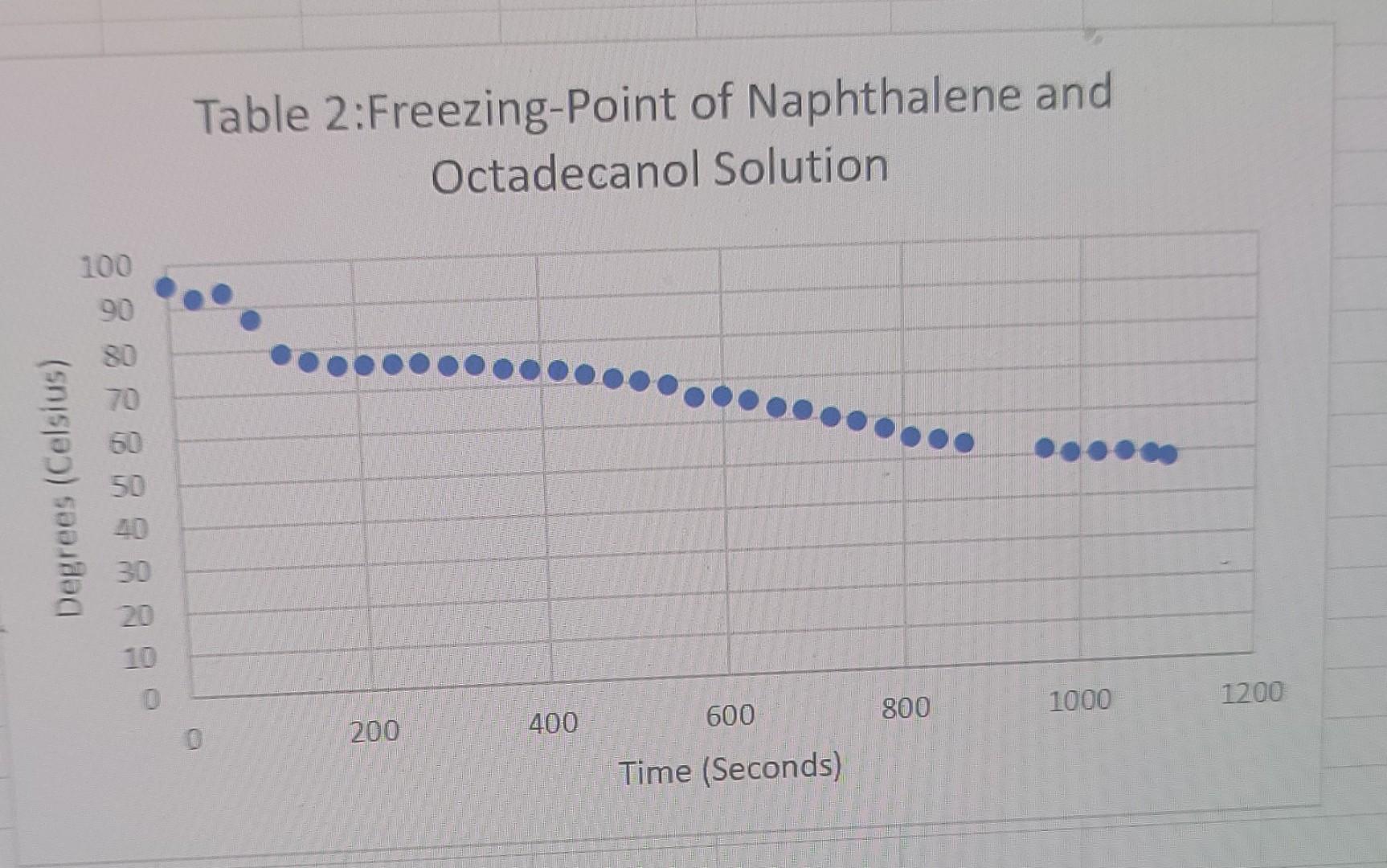
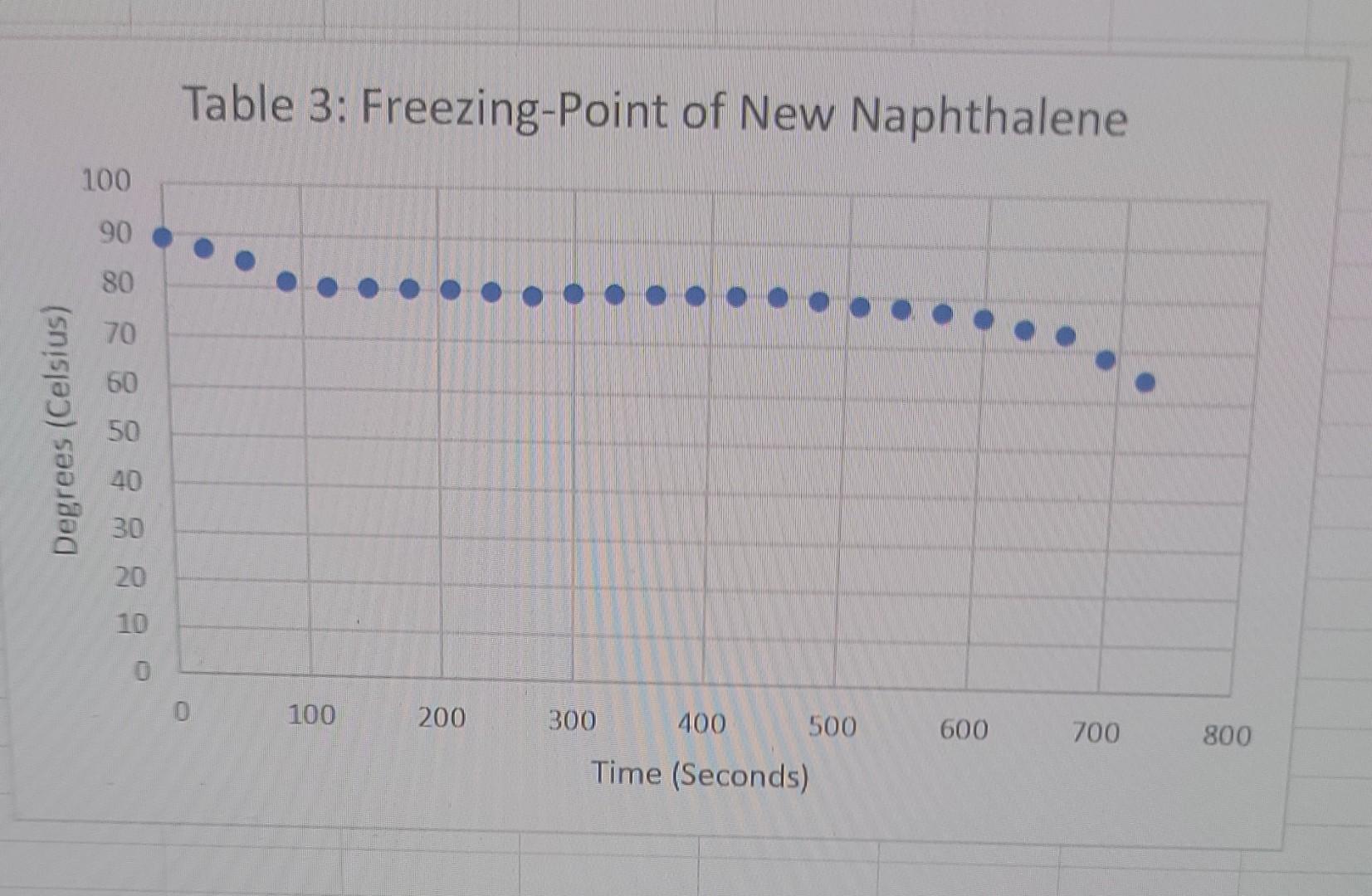
3rd trial 6.011g new naphthalene
4th trial 6.011g new naphthalene + 1.136 g unknown
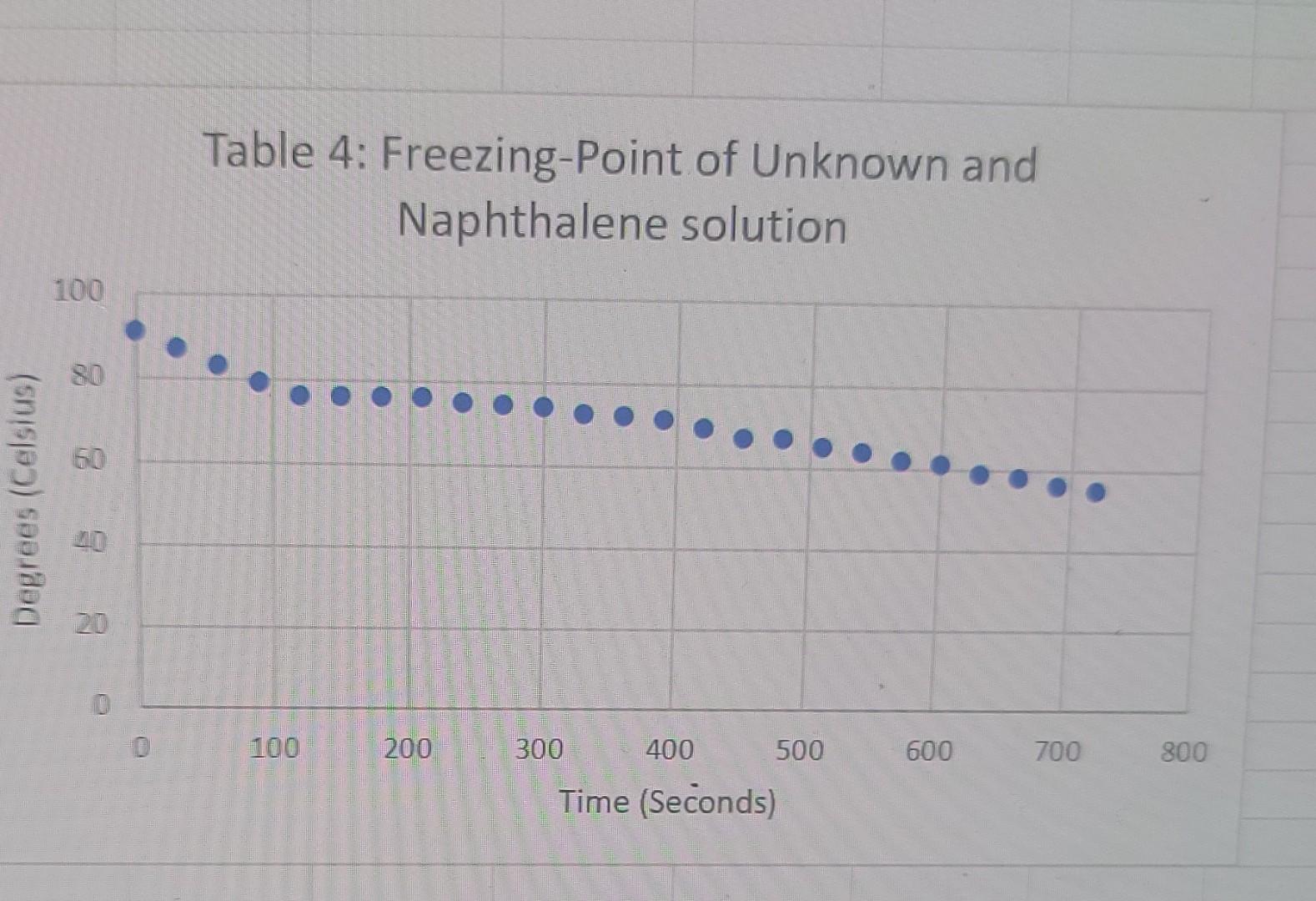
the end goal is to find the molar mass of the unknown if that helps. I don't know how to find Kf or what the m is supposed to be
liquid Freezing Point Terperature solid Time PROCESSING THE DATA 1. Determine the difference in freezing temperatures, AT, between the pure naphthalene (t1) and the mixture of naphthalene and Biphenyl. Use the formula, AT = T1 - T2, interpreting your graph. 2. Calculate the moles of biphenyl that you used. 3. Calculate molality (m), in mol/kg, of the biphenyl solution. 4. Using the formula, AT =i Kf.m, determine K for the naphthalene. 5. Determine the difference in freezing temperatures, AT, between the pure naphthalene (Ti) and the mixture of naphthalene and unknown. Use the formula, AT=T1-T2, interpreting your graph. 6. Using the formula, AT =i Kf.m, determine the molality of your unknown solution. 7. Calculate moles of Biphenyl or unknown solute, using the answer in Step 6 (in mol/kg) and the mass (in kg) of naphthalene solvent. Table 1: Freezing-Point of Naphthalene 100 Degrees (Celsius) - 8 8 8 8 8 8 8 8 8 8 100 200 300 400 500 600 700 800 900 Time (Seconds) Table 2:Freezing-Point of Naphthalene and Octadecanol Solution 100 90 80 Degrees (Celsius) 50 30 20 0 1000 1200 800 600 200 400 Time (Seconds) Table 3: Freezing-Point of New Naphthalene 100 90 80 70 60 Degrees (Celsius) 50 40 30 20 10 0 0 100 200 600 700 800 300 400 500 Time (Seconds) Table 4: Freezing-Point of Unknown and Naphthalene solution 100 Degrees Celsius) 80 60 20 20 100 200 300 400 500 600 700 800 Time (Seconds)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


