Question: hello, I am once again asking for you assitance. I am struggling with these questions, please don't just give the answers but also an explanation



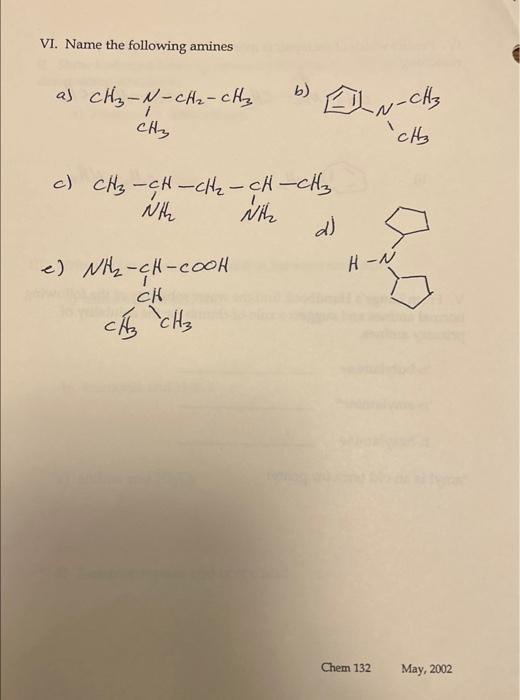
Amines 1. Build each of the following amines with the model kit. Draw the 3 dimensional structure in the space provided then state whether the amine is a primary, secondary or tertiary amine. a) N-methyl-1-aminoethane b) 2-aminopropane c) 1,3-diaminocyclobutane d) N-isopropylaminobenzene e) N,N-dimethyl-2,3-dichloroaniline II. Show hydrogen bonding between each pair by drawing the 3 dimensional structures: a) ethanol and aminoethane b) water and aniline III. Write the equation of the reaction between each pair: a) aminoethane and HCl b) ammonia and H2SO4 c) aniline and HNO3 d) 2-aminopropane and acetic acid IV. Predict which of each pair is more soluble and explain your reasoning. a) CH3NH2CH3 CH3NHCH3 b) CH3NH2 V. From Lange's Handbook find the water solubility of the following normal amines and suggest a rule-of-thumb for the solubility of primary amines. n-butylamine n-amylamine* n-hexylamine *amyl is an old term for pentyl VI. Name the following amines a) CH3NNCH2CH3CH3 b) c) e)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


