Question: hello. may someone explain in simple terms or another easier way than what this figure is representing? (a) (6) FIG. 2-9 Aggregation of nonpolar molecules
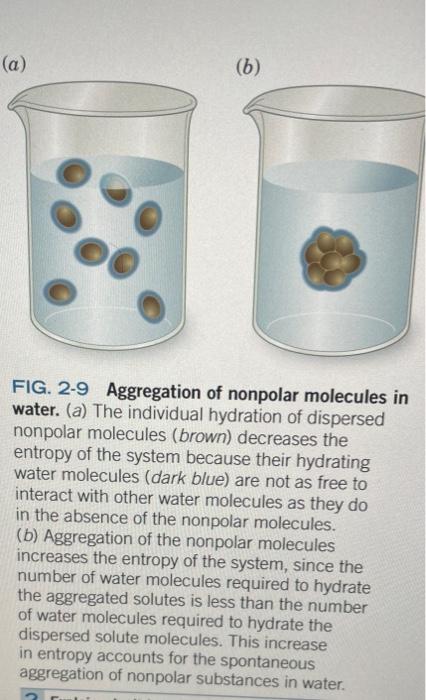
(a) (6) FIG. 2-9 Aggregation of nonpolar molecules in water. (a) The individual hydration of dispersed nonpolar molecules (brown) decreases the entropy of the system because their hydrating water molecules (dark blue) are not as free to interact with other water molecules as they do in the absence of the nonpolar molecules. (b) Aggregation of the nonpolar molecules increases the entropy of the system, since the number of water molecules required to hydrate the aggregated solutes is less than the number of water molecules required to hydrate the dispersed solute molecules. This increase in entropy accounts for the spontaneous aggregation of nonpolar substances in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


