Question: Hello Please help me for this question with polymath, do not send me other experts answers. 1A The following liquid-phase reactions were carried out in
Hello Please help me for this question with polymath, do not send me other experts answers.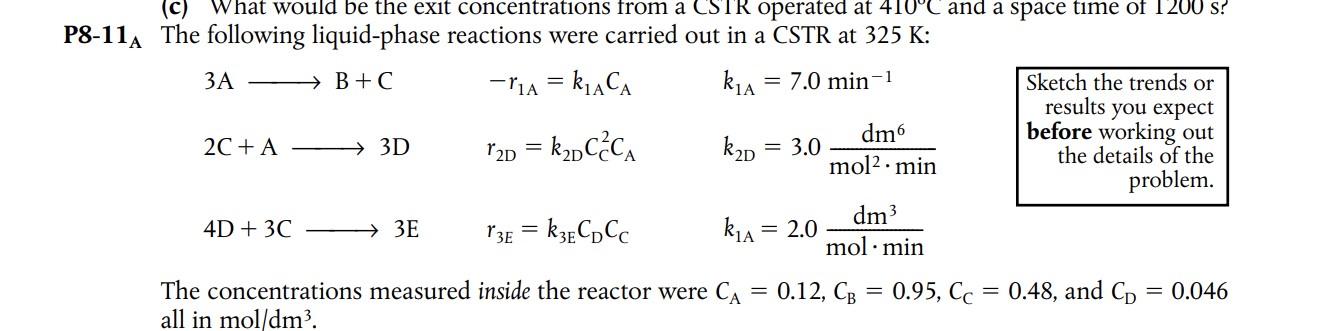

1A The following liquid-phase reactions were carried out in a CSTR at 325K : 3AB+C2C+A3D4D+3C3Er1A=k1ACAr2D=k2DCC2CAr3E=k3ECDCCk1A=7.0min1k2D=3.0mol2mindm6k1A=2.0molmindm3 \begin{tabular}{|r|} \hline Sketch the trends or \\ results you expect \\ before working out \\ the details of the \\ problem. \\ \hline \end{tabular} The concentrations measured inside the reactor were CA=0.12,CB=0.95,CC=0.48, and CD=0.046 all in mol/dm3. PFR. Now assume the reactions take place in the gas phase. Use the preceding data to plot the molar flow rate's selectivity and p as a function of PFR volume up to 400dm3. The pressure drop parameter is 0.001dm3. The total concentration entering the reactor is 0.2mol/dm3, and v0=95dm3/min. What are the values of S~D/E and S~C/D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


