Question: hello this is one assignment experiment please complete all with good handwritting number of dropts fo first generate the ecintact lens apperarance) 9. Clean the
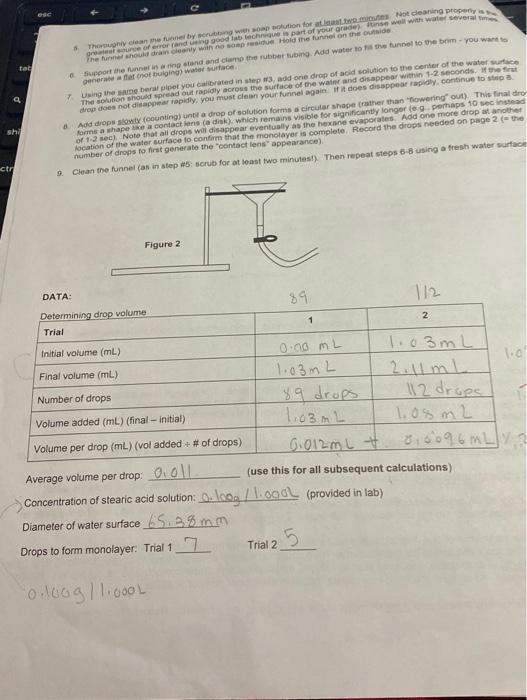

number of dropts fo first generate the "ecintact lens" apperarance) 9. Clean the funnel (as in step 45 : scrub for at least two minutest). Then repeat steps 65 using a fresh water surfac Average volume per drop: 0,011 (use this for all subsequent calculations) Concentration of stearic acid solution: 0. log/1.0gOL (provided in lab) Diameter of water surface 65,38mm Drops to form monolayer. Trial 1 Trial 2 ESTIMATING AVOGADRO'S NUMBER The base unit for counting very amall particles (such as aloms or moleoules) is the mole. The rumber of partcles making up a mole is known as Avogadro's number. The accubted value for this constant is 0.0221000 tho four wig fos.) More than twenty different types of experiments have been used to verify Avogadro s number, some of these erperiments frolid tayer that is one molecule thick on a water surface (called a monolay. This Some molecules have different properties at different regions of their structure. In this expenment you will use one wach molecule a fatty acid called stearic acid ( C14H20COOH). Fatty acids have long, hydrophobic ('water-fearing) chains attached to a hydrophilic ('water-loving') end. CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3CH2CH2CH2CH2CH2COOH=CH2(CH2)1COOH When these molecules contact water, they tend to become oriented so that the hydrophalic end interacts with the water. By introducing the stearic acid slowly, we can generate a single layer with this orientation, shown schematically in Figure 1: Safety: - Goggles must be worn for the entire experiment. - Hexane is a skin irritant and is harmful if swallowed or inhaled. Avoid contact and report spils immediately. - Do not invert the berai pipettes (the tip should be lower than the bulb) Procedure: The monolayer will be generated using a solution of stearic acid in hexane. The solution is added dropwise to a water. surface, forming a layer one molecule thick. The hexane evaporates, leaving behind the stearic acid monolayer. The monolayer formation is complete when an additional drop of stearic acid solution forms a disk on the water that looks like a contact lens and remains visible for more than a few seconds. Note that the funnel must be thoroughly scrubbed with soapy water bofore using. The presence of any dirt or oil can ruin experimental results. 1. Obtain a tube of stearic acid solution from your instructor. Record the concentration on page 2 (note the units). 2. Obtain a dry 10mL graduated cylinder and a narrow tip plastic beral pipet. You must use the same beral pipette for the entire experiment. Do not use this pipet with water. Do not add water to the cylinder! 3. Determine the volume of one drop of stearic acid solution as delivered by your beral pipet: fill the graduated cylinde past the first mark and record the initial volume. Then count the number of drops required to raise the liquid level by least 1.00mL. For example, you could start at 1.00mL and add enough drops to reach 2.00mL (or higher). Repe this once more. As always, you must record your data with the proper number of digits. number of dropts fo first generate the "ecintact lens" apperarance) 9. Clean the funnel (as in step 45 : scrub for at least two minutest). Then repeat steps 65 using a fresh water surfac Average volume per drop: 0,011 (use this for all subsequent calculations) Concentration of stearic acid solution: 0. log/1.0gOL (provided in lab) Diameter of water surface 65,38mm Drops to form monolayer. Trial 1 Trial 2 ESTIMATING AVOGADRO'S NUMBER The base unit for counting very amall particles (such as aloms or moleoules) is the mole. The rumber of partcles making up a mole is known as Avogadro's number. The accubted value for this constant is 0.0221000 tho four wig fos.) More than twenty different types of experiments have been used to verify Avogadro s number, some of these erperiments frolid tayer that is one molecule thick on a water surface (called a monolay. This Some molecules have different properties at different regions of their structure. In this expenment you will use one wach molecule a fatty acid called stearic acid ( C14H20COOH). Fatty acids have long, hydrophobic ('water-fearing) chains attached to a hydrophilic ('water-loving') end. CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3CH2CH2CH2CH2CH2COOH=CH2(CH2)1COOH When these molecules contact water, they tend to become oriented so that the hydrophalic end interacts with the water. By introducing the stearic acid slowly, we can generate a single layer with this orientation, shown schematically in Figure 1: Safety: - Goggles must be worn for the entire experiment. - Hexane is a skin irritant and is harmful if swallowed or inhaled. Avoid contact and report spils immediately. - Do not invert the berai pipettes (the tip should be lower than the bulb) Procedure: The monolayer will be generated using a solution of stearic acid in hexane. The solution is added dropwise to a water. surface, forming a layer one molecule thick. The hexane evaporates, leaving behind the stearic acid monolayer. The monolayer formation is complete when an additional drop of stearic acid solution forms a disk on the water that looks like a contact lens and remains visible for more than a few seconds. Note that the funnel must be thoroughly scrubbed with soapy water bofore using. The presence of any dirt or oil can ruin experimental results. 1. Obtain a tube of stearic acid solution from your instructor. Record the concentration on page 2 (note the units). 2. Obtain a dry 10mL graduated cylinder and a narrow tip plastic beral pipet. You must use the same beral pipette for the entire experiment. Do not use this pipet with water. Do not add water to the cylinder! 3. Determine the volume of one drop of stearic acid solution as delivered by your beral pipet: fill the graduated cylinde past the first mark and record the initial volume. Then count the number of drops required to raise the liquid level by least 1.00mL. For example, you could start at 1.00mL and add enough drops to reach 2.00mL (or higher). Repe this once more. As always, you must record your data with the proper number of digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


