Question: please help me with this section Phase Changes Each inclividual chemical (Whilie in its llquid state) has what is called a freering and a boiling
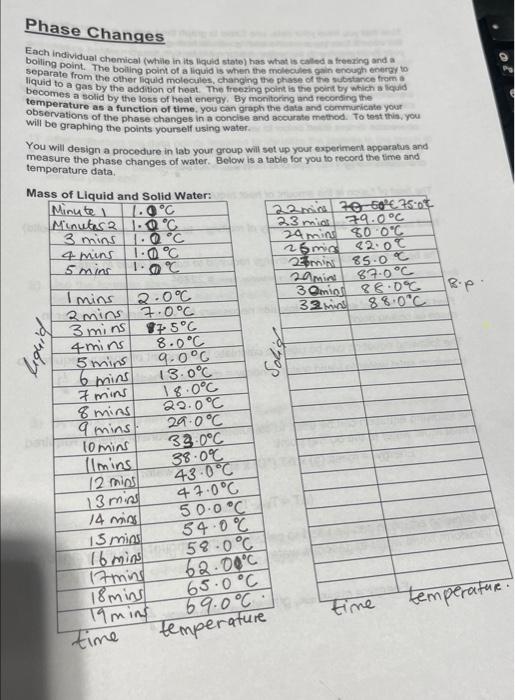


Phase Changes Each inclividual chemical (Whilie in its llquid state) has what is called a freering and a boiling point. The boling point of a llquid is when the molecules gain enough endergy to separate from the other liquid molecules, changing the phase of the mbstance fram of liquid to a gas by the addition of heat. The freezing point is the point by which a lequid becomes a solid by the loss of hoat energy. By monitoring and recording the temperature as a function of time, you can graph the data and commuricate yout observations of the phase changes in a conclse and accurate method. To test this, you will be graphing the points yourself using water. You will design a procedure in lab your group will sot up yout experiment apparabus and measure the phase changes of water. Below is a table for you to record the time and temperature data. Now that you have your data, use Excel to graph the heating curve starting with the water's treezing point and onding with its bolling point and plateau. Submit your Excel graph with the witton assignment for this experiment. With any kind of transformation, energy is needed and in this experiment the energy is heat energy, atso callod thermal energy. The amount of energy transferred can be calculated using a unit called a calorie. By knowing how many calories something has you can detemmine how much energy that substance can provide or consume. In order to find the amount of calories, there are different conversion factors listed below to use in order to find the amount of energy: The specific heat is the amount of heat required to ralse the temperature of 1.9 of a substance by 1.C; specific heat war =4.184gC1. The heat of fusion is the amount of heat required to melt 1,g of ice: heat fusion of water =334L. (energy of melting - to separate particles of a solid) The heat of vaporization is the energy required to convert liquid to gas; heatvaporizationwater=2260gL Find the amount of energy in kilojoules necessary for your sample of water to reach its boiling point. The following equation can be used to calculate the amount of energy required to transform solid water to liquid water: Energy = massice, g (heatusion of water, g4 ) The following equation can be used to calculated the amount of energy required to heat liquid water to the boiling point: Energy=mass(g)T(C)sp.heatofwater(gC1) (T=TfratTinisal) The following equation can be used to calculated the amount of energy needed transform liquid water to gaseous water. Energy = massilquid, g( heatvaporization of water, gf) Use these equations and constants to calculate the energy required in the processes below. Carefully consider which equation or combination of using full sentercopriate for the process you are asked to analyyte. Answer written answer. Calculated answers requiro a demonstration of the uning a analysis, a step-by-step calculation, and a final answer presented with the correct units and number of significant figures. Answer the following questions: 1) After collecting the data points required to visualize the plateau in the graph at the higher temperatures, we shut off the hot plate. Why? What would have happened if we had left the experimental sotup alone for several houts with the hot plate on? 2) Use the formula above to determine the total number of joules needed to melt the solid water AND heat the water to its boiling point: 3) By using the graph you created in Excel, how long did it take the water to reach its boiling point from its freezing point? 4) Why is your boiling point different than the stated boiling point of water? HINT: At what elevation was this experiment performed? Phase Changes Each inclividual chemical (Whilie in its llquid state) has what is called a freering and a boiling point. The boling point of a llquid is when the molecules gain enough endergy to separate from the other liquid molecules, changing the phase of the mbstance fram of liquid to a gas by the addition of heat. The freezing point is the point by which a lequid becomes a solid by the loss of hoat energy. By monitoring and recording the temperature as a function of time, you can graph the data and commuricate yout observations of the phase changes in a conclse and accurate method. To test this, you will be graphing the points yourself using water. You will design a procedure in lab your group will sot up yout experiment apparabus and measure the phase changes of water. Below is a table for you to record the time and temperature data. Now that you have your data, use Excel to graph the heating curve starting with the water's treezing point and onding with its bolling point and plateau. Submit your Excel graph with the witton assignment for this experiment. With any kind of transformation, energy is needed and in this experiment the energy is heat energy, atso callod thermal energy. The amount of energy transferred can be calculated using a unit called a calorie. By knowing how many calories something has you can detemmine how much energy that substance can provide or consume. In order to find the amount of calories, there are different conversion factors listed below to use in order to find the amount of energy: The specific heat is the amount of heat required to ralse the temperature of 1.9 of a substance by 1.C; specific heat war =4.184gC1. The heat of fusion is the amount of heat required to melt 1,g of ice: heat fusion of water =334L. (energy of melting - to separate particles of a solid) The heat of vaporization is the energy required to convert liquid to gas; heatvaporizationwater=2260gL Find the amount of energy in kilojoules necessary for your sample of water to reach its boiling point. The following equation can be used to calculate the amount of energy required to transform solid water to liquid water: Energy = massice, g (heatusion of water, g4 ) The following equation can be used to calculated the amount of energy required to heat liquid water to the boiling point: Energy=mass(g)T(C)sp.heatofwater(gC1) (T=TfratTinisal) The following equation can be used to calculated the amount of energy needed transform liquid water to gaseous water. Energy = massilquid, g( heatvaporization of water, gf) Use these equations and constants to calculate the energy required in the processes below. Carefully consider which equation or combination of using full sentercopriate for the process you are asked to analyyte. Answer written answer. Calculated answers requiro a demonstration of the uning a analysis, a step-by-step calculation, and a final answer presented with the correct units and number of significant figures. Answer the following questions: 1) After collecting the data points required to visualize the plateau in the graph at the higher temperatures, we shut off the hot plate. Why? What would have happened if we had left the experimental sotup alone for several houts with the hot plate on? 2) Use the formula above to determine the total number of joules needed to melt the solid water AND heat the water to its boiling point: 3) By using the graph you created in Excel, how long did it take the water to reach its boiling point from its freezing point? 4) Why is your boiling point different than the stated boiling point of water? HINT: At what elevation was this experiment performed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


