Question: help 7) Given this unbalanced reaction: C.H.N + O2 CO2 + H2O + N2, and stoichiometric oxygen, what mole fraction of the product stream is
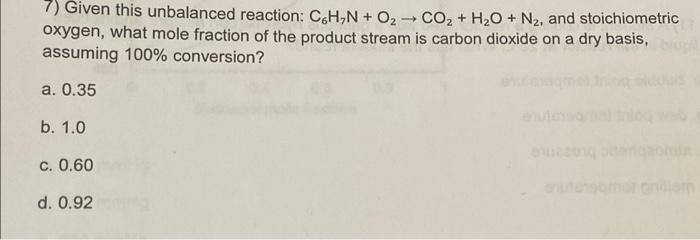
7) Given this unbalanced reaction: C.H.N + O2 CO2 + H2O + N2, and stoichiometric oxygen, what mole fraction of the product stream is carbon dioxide on a dry basis, assuming 100% conversion? a. 0.35 b. 1.0 c. 0.60 d. 0.92
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


