Question: Help! answer correctly and clearly ill thumbs up! Part A: Boiling Point of an Unknown Liquid H Unknown Liquid: M Boiling point of the liquid

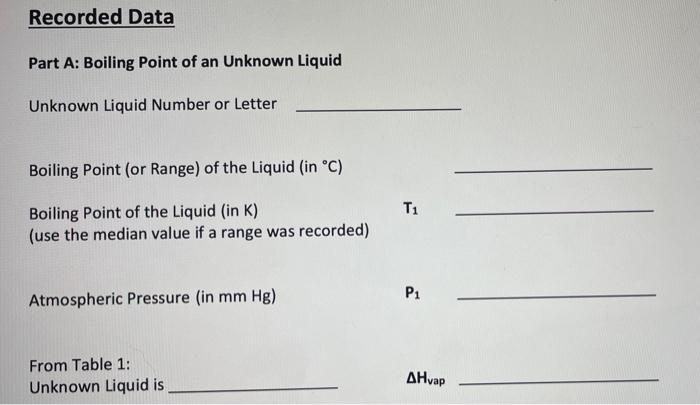
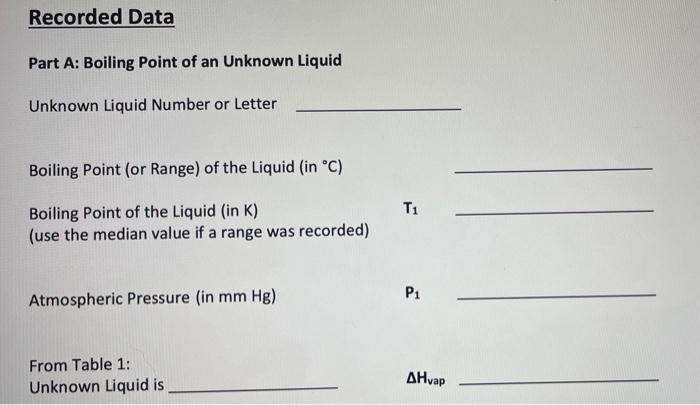

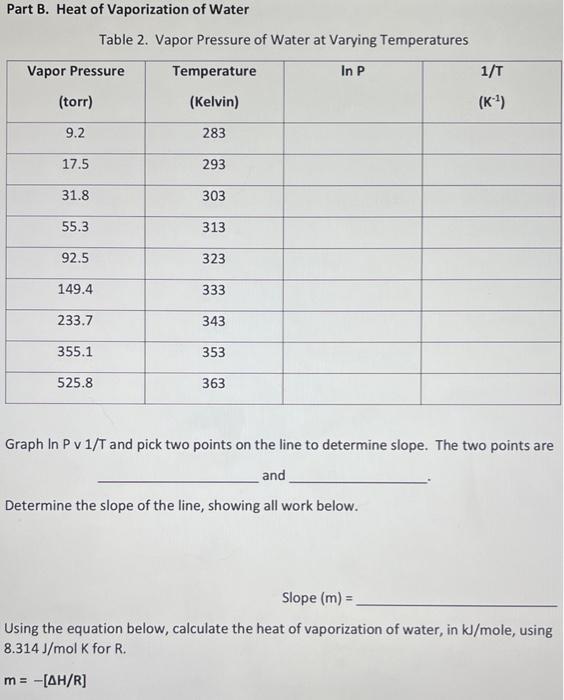

Part A: Boiling Point of an Unknown Liquid H Unknown Liquid: M Boiling point of the liquid in celsius : 64.9 - 65.2 C Boiling point of the liquid in Kelvin (use the median value if a range was recorded) : _ Atmospheric Pressure (mmHg) = 761.21 mmHg Once you identify what the unknown liquid is look up the AHvap in the table in the lab manual and use it for ti Remember to convert your units. The value of Ris in terms of Joules and AHvap is in terms of kilojoules. Recorded Data Part A: Boiling Point of an Unknown Liquid Unknown Liquid Number or Letter Boiling Point (or Range) of the Liquid (in C) Boiling Point of the Liquid (in K) (use the median value if a range was recorded) Pi Atmospheric Pressure (in mm Hg) From Table 1: Unknown Liquid is AHvap Recorded Data Part A: Boiling Point of an Unknown Liquid Unknown Liquid Number or Letter Boiling Point (or Range) of the Liquid (in C) Boiling Point of the Liquid (in K) (use the median value if a range was recorded) Pi Atmospheric Pressure (in mm Hg) From Table 1: Unknown Liquid is AHvap Calculated Data Determine the vapor pressure of your unknown liquid at 295 k using the Clausius- Clapeyron equation. Show all work in the spaces provided. Vapor Pressure of Unknown at 295 K Part B. Heat of Vaporization of Water Table 2. Vapor Pressure of Water at Varying Temperatures Vapor Pressure Temperature In P 1/T (torr) (Kelvin) (K) 9.2 283 17.5 293 31.8 303 55.3 313 92.5 323 149.4 333 233.7 343 355.1 353 525.8 363 Graph In P v1/T and pick two points on the line to determine slope. The two points are and Determine the slope of the line, showing all work below. Slope (m) = Using the equation below, calculate the heat of vaporization of water, in kJ/mole, using 8.314 J/mol K for R. m= -[AH/R] AHvap for water is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


