Question: help asap please The structure shown below is has a very high pKa because the conjugate base is very unstable. Using the provided resonance structures,
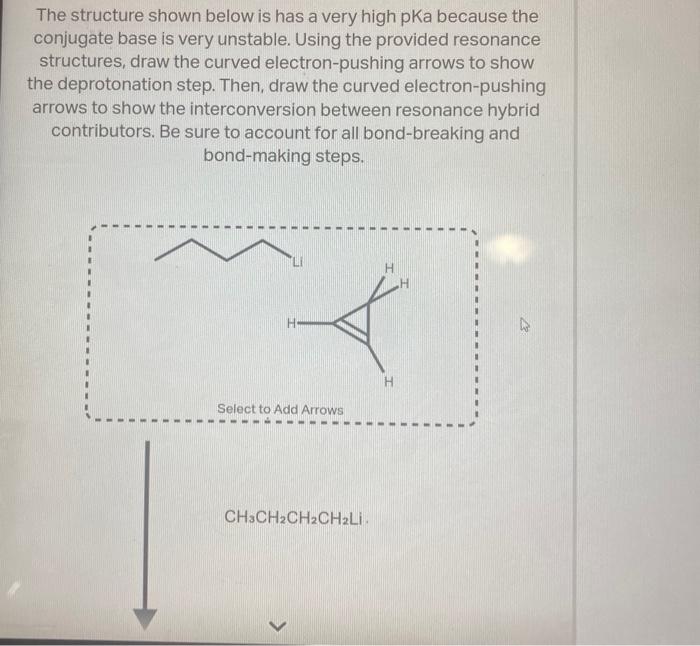
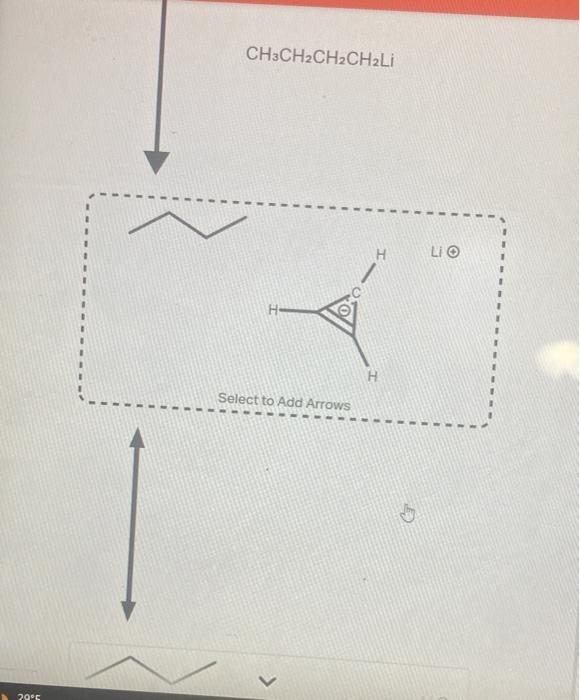
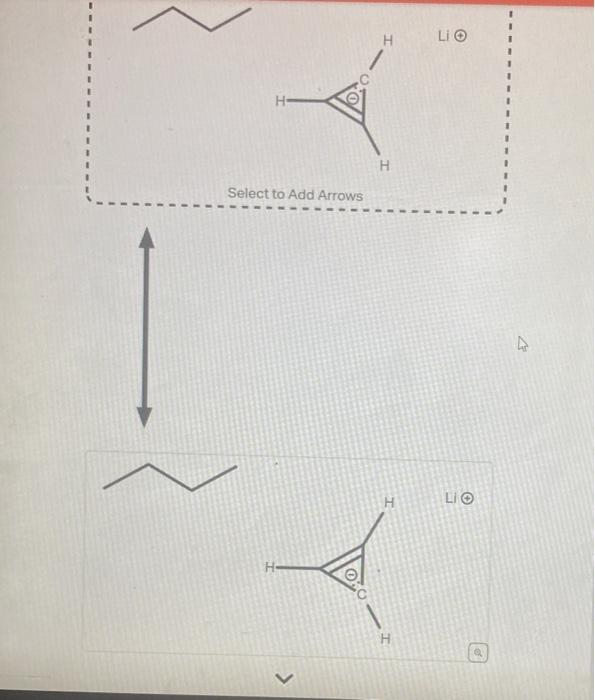
The structure shown below is has a very high pKa because the conjugate base is very unstable. Using the provided resonance structures, draw the curved electron-pushing arrows to show the deprotonation step. Then, draw the curved electron-pushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps. CH3CH2CH2CH2Li Select to Add Arrows Li Select to Add Arrows Li)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


