Question: help asap plz 3. Consider a cylindrical batch reactor that has one end fitted with a frictionless piston attached to a spring. The gas phase
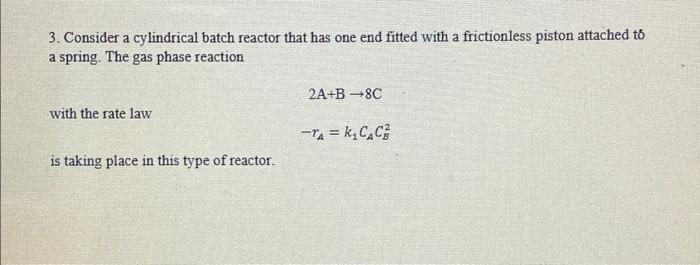
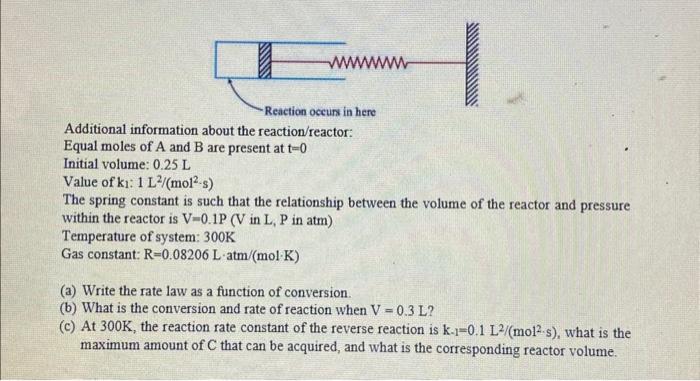
3. Consider a cylindrical batch reactor that has one end fitted with a frictionless piston attached to a spring. The gas phase reaction 2A+B8C with the rate law rA=k1CACB2 is taking place in this type of reactor. Additional information about the reaction/reactor: Equal moles of A and B are present at t=0 Initial volume: 0.25L The spring constant is such that the relationship between the volume of the reactor and pressure within the reactor is V=0.1P ( V in L,P in atm) Temperature of system: 300K Gas constant: R=0.08206Latm/(molK) (a) Write the rate law as a function of conversion. (b) What is the conversion and rate of reaction when V=0.3L ? (c) At 300K, the reaction rate constant of the reverse reaction is k1=0.1L2/(mol2s), what is the maximum amount of C that can be acquired, and what is the corresponding reactor volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


