Question: Help! Consider the gas-phase reaction between nitric oxide (NO) and bromine (Br2) at 273C : 2NO(g)+Br2(g)2NOBr(g) The following data for the initial rate of disappearance
Help! 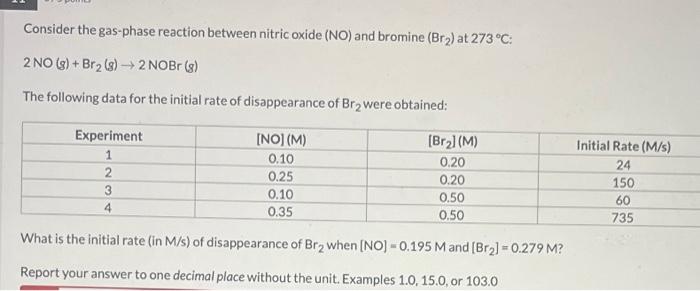

Consider the gas-phase reaction between nitric oxide (NO) and bromine (Br2) at 273C : 2NO(g)+Br2(g)2NOBr(g) The following data for the initial rate of disappearance of Br2 were obtained: What is the initial rate (in M/s ) of disappearance of Br2 when [NO]=0.195M and [Br2]=0.279M ? Report your answer to one decimal place without the unit. Examples 1.0,15.0, or 103.0
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


