Question: Try to construct a molar balance table by showing the initial, change, and final in mol for component A, B, and C. Then, try to
 Try to construct a molar balance table by showing the initial, change, and final in mol for component A, B, and C. Then, try to figure out the mole fraction for component A, B, and C. Also, think about the vant Hoff equation for this problem. Also, when considering temperature ranges, focus on the range of 240-320K.
Try to construct a molar balance table by showing the initial, change, and final in mol for component A, B, and C. Then, try to figure out the mole fraction for component A, B, and C. Also, think about the vant Hoff equation for this problem. Also, when considering temperature ranges, focus on the range of 240-320K.
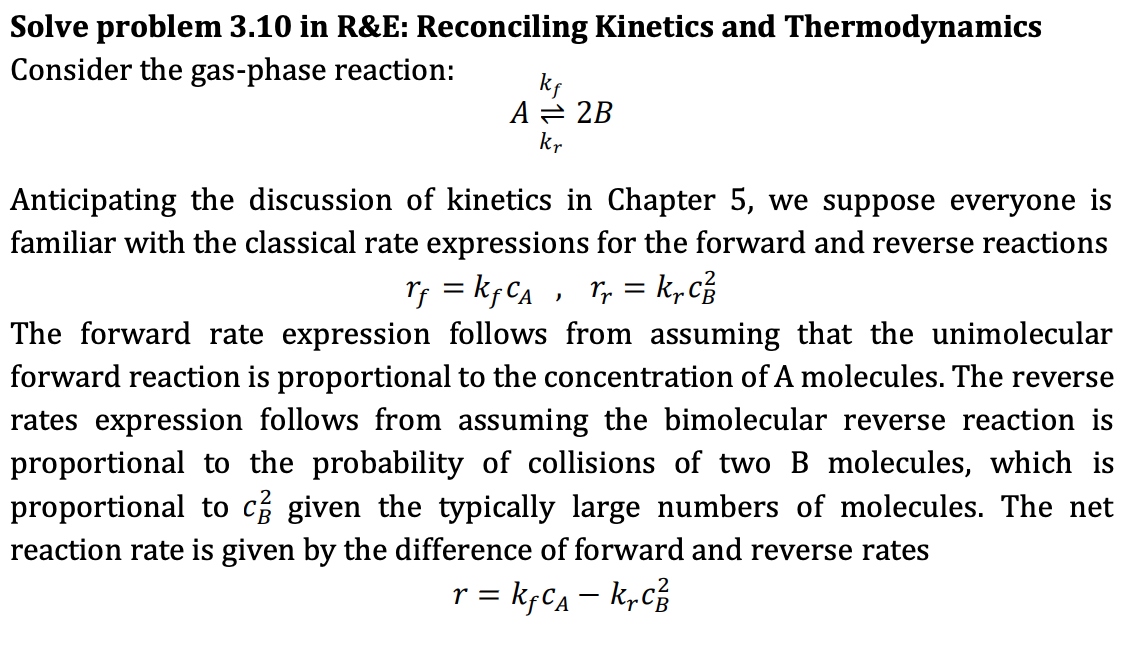
Solve problem 3.10 in R&E: Reconciling Kinetics and Thermodynamics Consider the gas-phase reaction: ke A = 2B kr Anticipating the discussion of kinetics in Chapter 5, we suppose everyone is familiar with the classical rate expressions for the forward and reverse reactions rf = kf Ca , rr = k, The forward rate expression follows from assuming that the unimolecular forward reaction is proportional to the concentration of A molecules. The reverse rates expression follows from assuming the bimolecular reverse reaction is proportional to the probability of collisions of two B molecules, which is proportional to c given the typically large numbers of molecules. The net reaction rate is given by the difference of forward and reverse rates r = kyca - krc Solve problem 3.10 in R&E: Reconciling Kinetics and Thermodynamics Consider the gas-phase reaction: ke A = 2B kr Anticipating the discussion of kinetics in Chapter 5, we suppose everyone is familiar with the classical rate expressions for the forward and reverse reactions rf = kf Ca , rr = k, The forward rate expression follows from assuming that the unimolecular forward reaction is proportional to the concentration of A molecules. The reverse rates expression follows from assuming the bimolecular reverse reaction is proportional to the probability of collisions of two B molecules, which is proportional to c given the typically large numbers of molecules. The net reaction rate is given by the difference of forward and reverse rates r = kyca - krc
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


