Question: help Equlibrium Using the Equilibrium Constant Part A The reversibie chemical resctoo A+B=C+D intaty any A and B are presert, each in 2.00M. What is
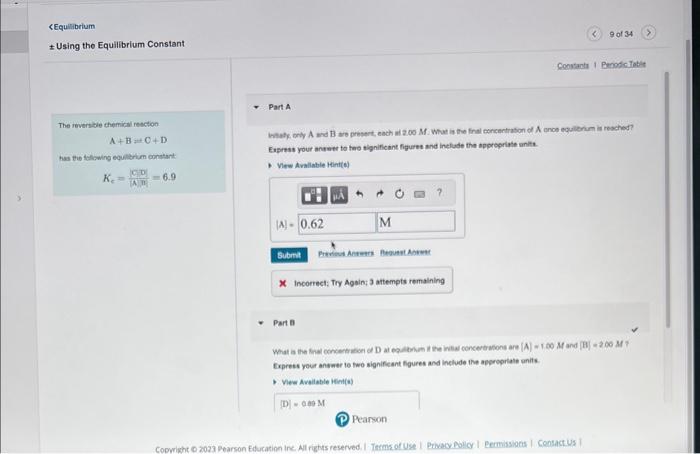
Equlibrium Using the Equilibrium Constant Part A The reversibie chemical resctoo A+B=C+D intaty any A and B are presert, each in 2.00M. What is the fral exrcectrason of A once equlenum is reached? thas the tilising equilitien onntart Kc=WGD]=6.9 x Incoerect: Try Againy 3 attempts remaining Pert al Exprest your answer to two significant foures and inelude the approprlate anits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


