Question: help fill in the chart please, im confused A. Determination of the Orders of Reaction and the Rate Pipette 1.) Use small beakers or graduated
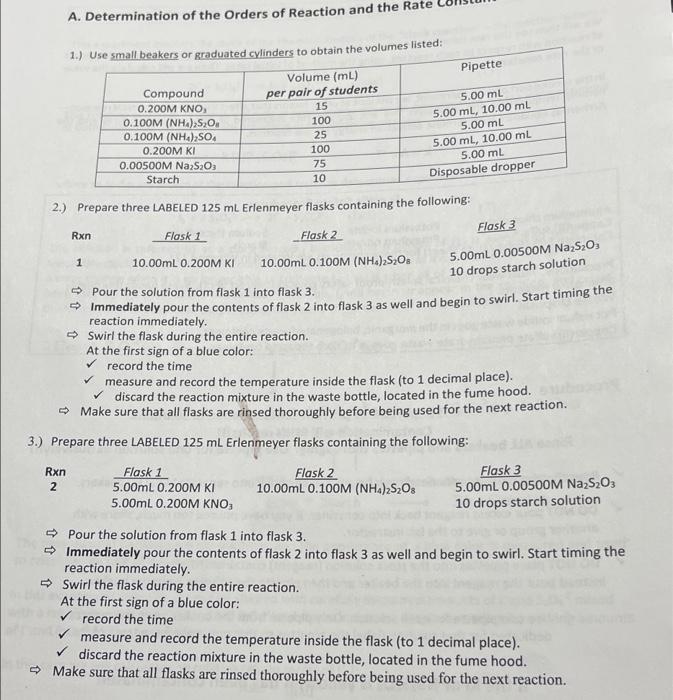

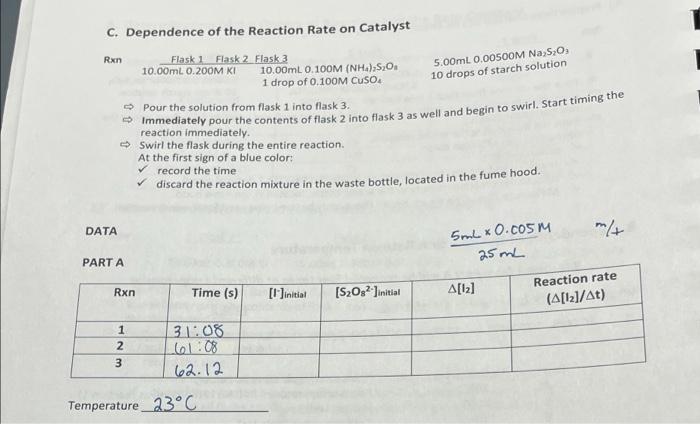
A. Determination of the Orders of Reaction and the Rate Pipette 1.) Use small beakers or graduated cylinders to obtain the volumes listed: Volume (mL) Compound per pair of students 0.200M KNO 15 0100M (NH4)2SO4 100 0.100M (NH4),SO 25 0.200M KI 100 0.00500M Na2S2O3 75 Starch 10 5.00 mL 5.00 mL, 10.00 mL 5.00 mL 5.00 mL, 10.00 mL 5.00 ml Disposable dropper 2.) Prepare three LABELED 125 ml Erlenmeyer flasks containing the following: Flask 3 Rxn Flask 1 Flask 2 1 10.00mL 0.200M KI 10.00mL 0.100M (NH.) S.O. 5.00mL 0.00500M Na2S20 10 drops starch solution Pour the solution from flask 1 into flask 3. - Immediately pour the contents of flask 2 into flask 3 as well and begin to swirl. Start timing the reaction immediately Swirl the flask during the entire reaction. At the first sign of a blue color: record the time measure and record the temperature inside the flask (to 1 decimal place). discard the reaction mixture in the waste bottle, located in the fume hood. Make sure that all flasks are rinsed thoroughly before being used for the next reaction. 3.) Prepare three LABELED 125 mL Erlenmeyer flasks containing the following: Rxn 2 Flask 1 5.00mL 0.200M KI 5.00mL 0.200M KNO Flask 2 10.00mL 0.100M (NH4)2S203 Flask 3 5.00mL 0.00500M Na2S2O3 10 drops starch solution -> Pour the solution from flask 1 into flask 3. - Immediately pour the contents of flask 2 into flask 3 as well and begin to swirl. Start timing the reaction immediately. Swirl the flask during the entire reaction. At the first sign of a blue color: record the time measure and record the temperature inside the flask (to 1 decimal place). discard the reaction mixture in the waste bottle, located in the fume hood. - Make sure that all flasks are rinsed thoroughly before being used for the next reaction. Run 3 3 4.) Prepare three LABELED 125 ml Erlenmeyer fiasks containing the following Flask 1 Flask 2 Elas 10.00ml 0.200M KI SOOry. O 100M (44) Da S.OOML00500M SO. 5.00mL 0.100M (NH) SO. 10 drops starch solution - Pour the solution from flask 1 into flask 3. Immediately pour the contents of flask 2 into flask 3 as well and begin to swiat. Szarz timing the reaction immediately Swirl the flask during the entire reaction. At the first sign of a blue color: record the time measure and record the temperature inside the flask (to 1 decimal place). discard the reaction mixture in the waste bottle, located in the fume hood, Make sure that all flasks are rinsed thoroughly before being used for the next reaction. 1 B. Dependence of the Reaction Rate on Temperature Reaction 1 from Part A will be repeated four times at different temperatures. Rxn Flask 1 Flask 2 Flask 3 10.00mL 0.200M KI 10.00mL 0.100M (NH).S.O. 5.00mL. 0.00500M Na,5,0 10 drops of starch solution Rxn Temperature **Method(please read below) 20c Room temperature (This is the same as reaction 1) 40C Hot tap water 10 C Cold tap water (little ice) 7 o'c Ice with a little tap water 4 5 6 *Method** Temperatures are approximate. The three flasks do NOT have to be at the listed temperature exactly, but they all must be at the same temperature (0.5c). Prepare a water bath (using a bin) at the appropriate temperature. Place all three flasks into the bath and wait for a constant temperature to be obtained in each flask. Procedure for rxn 5-7: - When the solutions reach the appropriate temperature record the temperature and pour the solution from flask 1 into flask 3. Immediately pour the contents of flask 2 into flask 3 as well and begin to swirl. Start timing the reaction immediately. Swirl the flask during the entire reaction. -> When the solution turns dark blue: record the time measure and reco the temperature inside the flask (to 1 decimal place). Calculate an average temperature for the reaction. Make sure that all flasks are rinsed thoroughly before being used for the next reaction. 1 5.00mL 0.0050OM Na 5,0 10 drops of starch solution C. Dependence of the Reaction Rate on Catalyst Rxn Flask 1 Flask 2 Flask 3 10.00ml. 0.200M KI 10,00ml. 0, 100M (NH4)2S2O 1 drop of 0.100M CUSO. - Pour the solution from flask 1 into flask 3. > Immediately pour the contents of flask 2 into flask 3 as well and begin to swirl. Start timing the reaction immediately. Swirl the flask during the entire reaction. At the first sign of a blue color: record the time discard the reaction mixture in the waste bottle, located in the fume hood. ATA mt 5ml x 0.com 25mL PARTA Rxn Time (s) [1]initial [S20s? Jinitial A[12] Reaction rate (A[12]/At) 1 2 3 31:08 601:08 62.12 Temperature_23C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


