Question: help! Lab Period 2 1) Weigh out an empty beaker 2) Transfer the product along with your filter paper to the beaker and weigh it
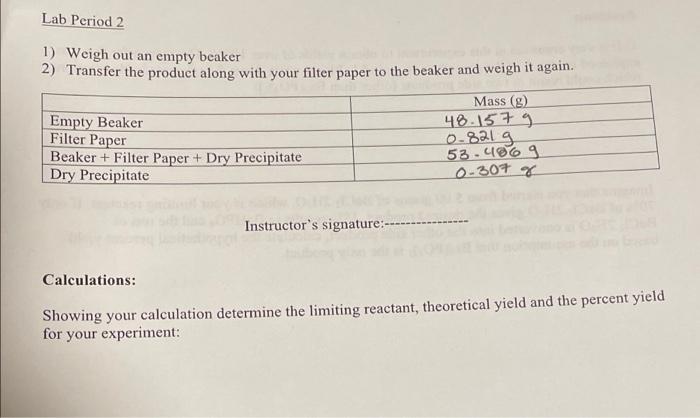

Lab Period 2 1) Weigh out an empty beaker 2) Transfer the product along with your filter paper to the beaker and weigh it again. Mass (g) Empty Beaker 48.1579 Filter Paper 0.821 g Beaker + Filter Paper + Dry Precipitate 53.4069 Dry Precipitate 0-307 g Instructor's signature:--- Calculations: Showing your calculation determine the limiting reactant, theoretical yield and the percent yield for your experiment: Problems 1) It is very common in this experiment to obtain yields of over 100%. The reason for this was explained in step 5 of the procedure in period 1. Please write down the unwanted side reactions, which could result in two side products (other than Ba3(PO4)2): Imagine that you started from 5.00 grams of BaCl2.2H,0, which is your limiting reactant. If only 70% of BaCl2.2H20 gives the desired product, 18% results in BaHPO, and the rest of BaCl2.2H20 is converted into the other side product, calculate the hypothetical percent yield based on the assumption that Ba,(PO4)2 is the only product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


