Question: Calculation: Showing your calculation determine the limiting reactant, theoretical yield and the percent yield for your experiment: 1) Weigh out an empty beaker 2) Transfer
Showing your calculation determine the limiting reactant, theoretical yield and the percent yield for your experiment:
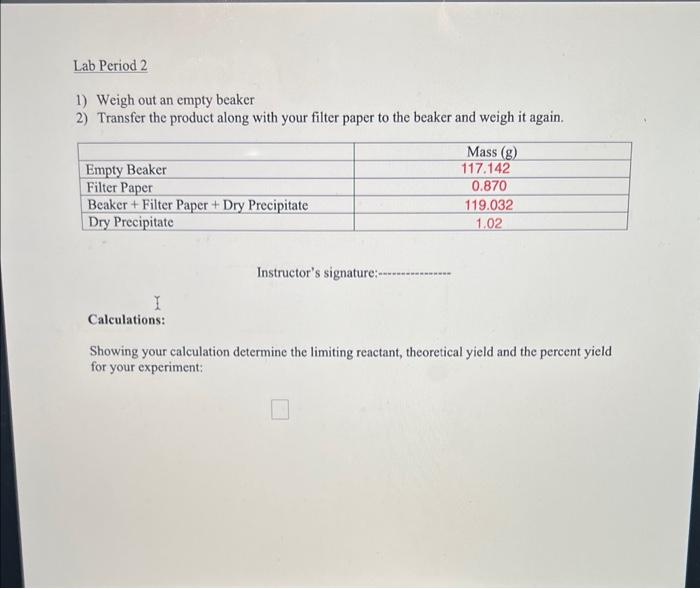
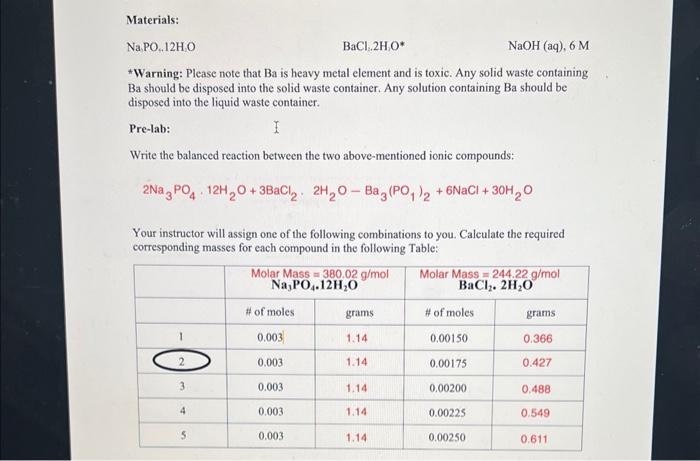
1) Weigh out an empty beaker 2) Transfer the product along with your filter paper to the beaker and weigh it again. Instructor's signature: Calculations: Showing your calculation determine the limiting reactant, theoretical yield and the percent yield for your experiment: *Warning: Please note that Ba is heavy metal element and is toxic. Any solid waste containing Ba should be disposed into the solid waste container. Any solution containing Ba should be disposed into the liquid waste container. Pre-lab: Write the balanced reaction between the two above-mentioned ionic compounds: 2Na3PO412H2O+3BaCl22H2OBa3(PO1)2+6NaCl+30H2O Your instructor will assign one of the following combinations to you. Calculate the required corresponding masses for each compound in the following Table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


