Question: help me answer these please 4. Consider the steps you followed to calculate the mass % concentration of vinegar and rationalize how will the following
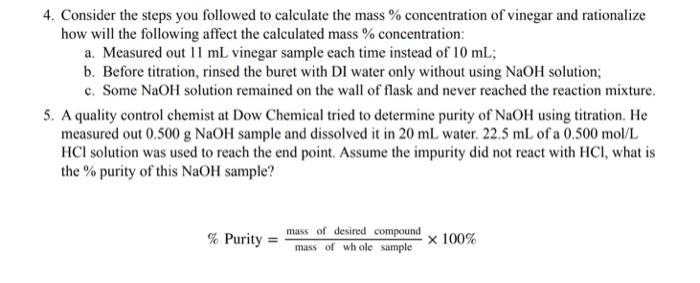
4. Consider the steps you followed to calculate the mass % concentration of vinegar and rationalize how will the following affect the calculated mass \% concentration: a. Measured out 11mL vinegar sample each time instead of 10mL; b. Before titration, rinsed the buret with DI water only without using NaOH solution; c. Some NaOH solution remained on the wall of flask and never reached the reaction mixture. 5. A quality control chemist at Dow Chemical tried to determine purity of NaOH using titration. He measured out 0.500gNaOH sample and dissolved it in 20mL water. 22.5mL of a 0.500mol/L HCl solution was used to reach the end point. Assume the impurity did not react with HCl, what is the % purity of this NaOH sample? %Purity=massofwholesumplemassofdesiredcompound100%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


