Question: Help me answer this 2 please 30. A 10.0mL sample of acidified sodium oxalate is titrated with a 0.0200 mol/L solution of potassium permanganate, as

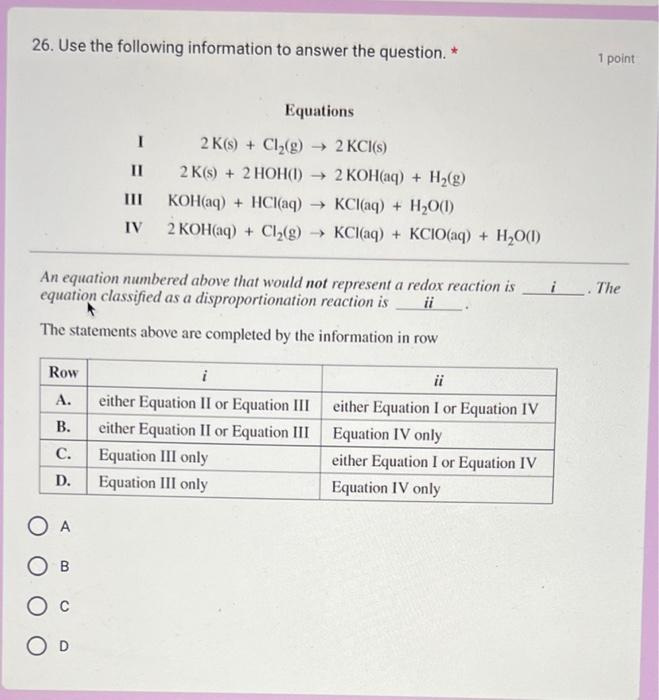
30. A 10.0mL sample of acidified sodium oxalate is titrated with a 0.0200 mol/L solution of potassium permanganate, as represented by the following net equation. The concentration of sodium oxalate in the original sample is mol/L 2MnO4(aq)+5OOCCOO2(aq)+16H+(aq)2Mn2+(aq)+10CO2(g)+8H2O(I) The initial and final burette readings for the titration are shown below. 26. Use the following information to answer the question. * 1 point Equations I2K(s)+Cl2(g)2KCl(s)II2K(s)+2HOH(I)2KOH(aq)+H2(g)IIIKOH(aq)+HCl(aq)KCl(aq)+H2O(I)IV2KOH(aq)+Cl2(g)KCl(aq)+KClO(aq)+H2O(I) An equation numbered above that would not represent a redox reaction is i. The equation classified as a disproportionation reaction is ii The statements above are completed by the information in row
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


