Question: please help me answer these chem questions i really, really need some help please! and i dont have many questions left that's why im using




![kale smoothie has a [OH]=0.0000030mol/L. What is its pH ? 36. Calculate](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8486a495d8_49766f84869b97d7.jpg)


![A. [H3O+(aq)]1.0107mol/L C D B A 37. A student performs 5 indicator](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8486c77b15_49966f8486be27e7.jpg)
please help me answer these chem questions i really, really need some help please! and i dont have many questions left that's why im using this format, thank you so much!! i've already answered them, please just check them over and please let me know if there are any wrong answers i've put, thank you so much!!
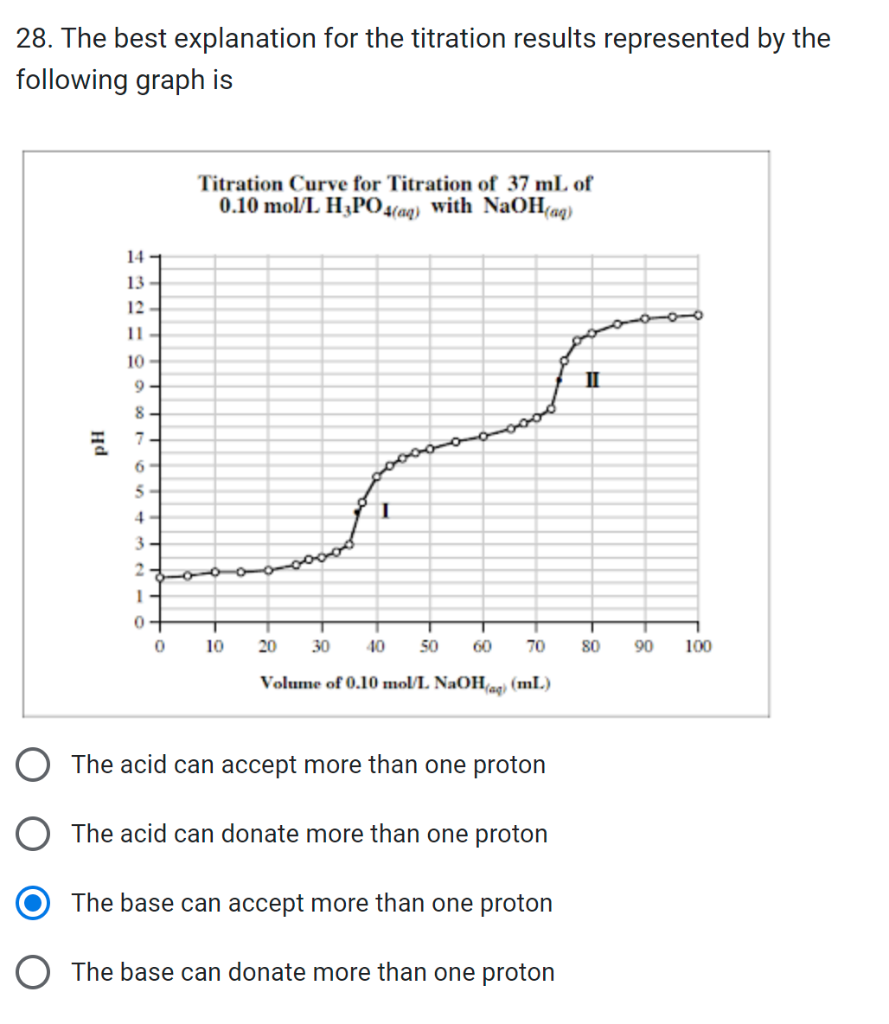
30. A kale smoothie has a [OH]=0.0000030mol/L. What is its pH ? 36. Calculate the pH of a solution with a hydronium ion concentration of 0.00128mol/L 24. For an acidic solution, which of the following is true? * A. [H3O+(aq)]1.0107mol/L C D B A 37. A student performs 5 indicator tests on a solution in a lab. Based on the results below, what is the pH of the solution being tested? a) Indigo carmine turns blue b) Thymol blue turns yellow c) Blue litmus turns red d) Chlorophenol red turns orange e) Phenol red turns yellow 5.6 28. The best explanation for the titration results represented by the following graph is The acid can accept more than one proton The acid can donate more than one proton The base can accept more than one proton The base can donate more than one proton 39. A student titrated a 10.0mL sample of nitric acid with a 1.50mol/L sodium hydroxide solution in the presence of an indicator. Calculate the concentration in mol/L of the nitric acid using the data in the following table. 40. How many grams of H3PO4 are in 180mL of a 2.50M solution of H3PO4 ? 15 Honin miloh annaantratad HCI 110MM ic naadad in tha nranaration of 21. What is the average volume of NaOH that should be used to determine the concentration of the hydrochloric acid? Titration of 15.00mL of Hydrochloric Acid, HCl(oq), with 0.100mol/L Sodium Hydroxide, NaOH(oq) 10.03mL 25.51mL 15.38mL 10.14mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


