Question: help me asap! Directions: Answer each question to the best of your ability. Be sure to show your thought process. Budget your time wisely -
help me asap!
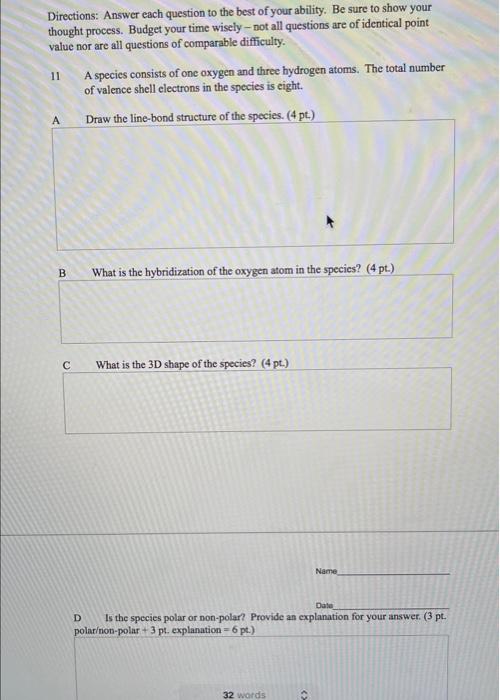
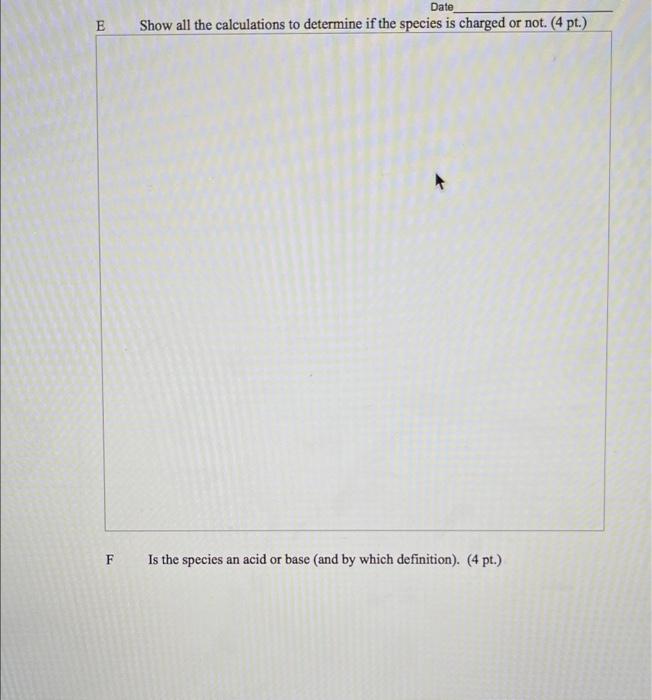
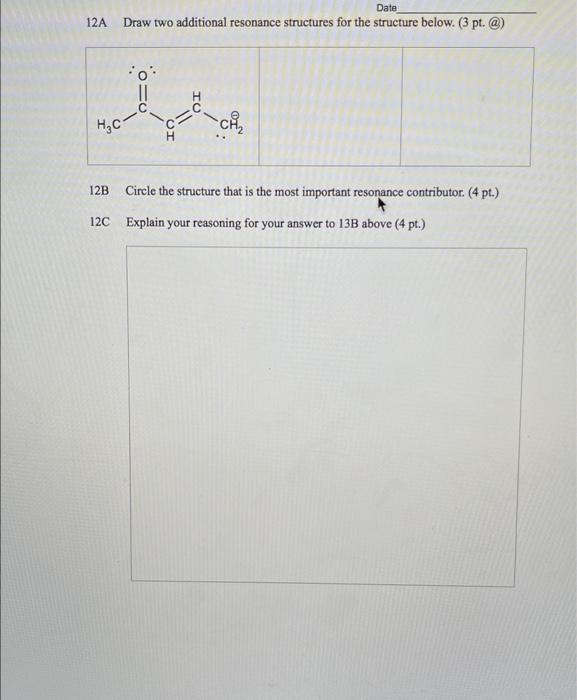
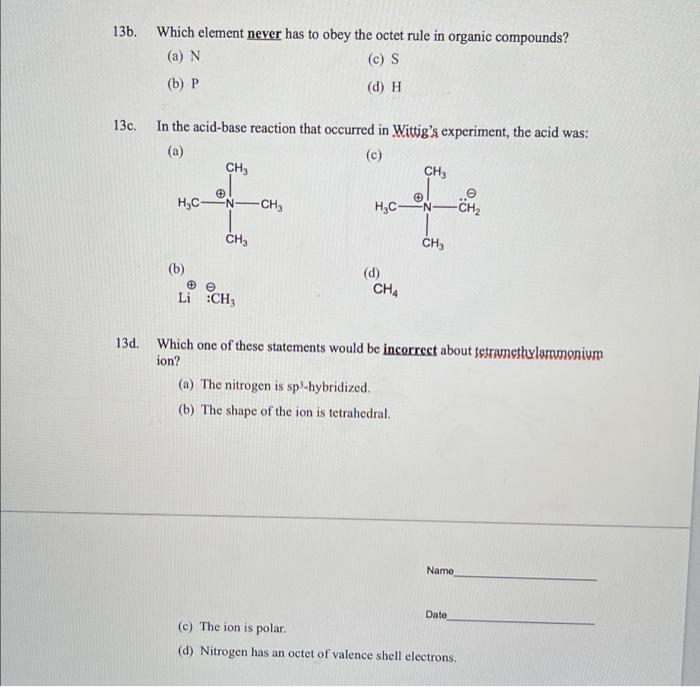
Directions: Answer each question to the best of your ability. Be sure to show your thought process. Budget your time wisely - not all questions are of identical point value nor are all questions of comparable difficulty. 11 A species consists of one oxygen and three hydrogen atoms. The total number of valence shell electrons in the species is eight. Draw the line-bond structure of the species. (4 pt.) B What is the hybridization of the oxygen atom in the species? (4 pt.) What is the 3D shape of the species? (4 pt.) Name Date D Is the species polar or non-polar? Provide an explanation for your answer. (3 pt. polaron-polar + 3 pl. explanation = 6 pt.) 32 words Date E Show all the calculations to determine if the species is charged or not. (4 pt.) F Is the species an acid or base (and by which definition). (4 pt.) 12A Date Draw two additional resonance structures for the structure below. (3 pt. @) :o HC- "ch 12B Circle the structure that is the most important resonance contributor. (4 pt.) 12C Explain your reasoning for your answer to 13B above (4 pt.) 13b. Which element never has to obey the octet rule in organic compounds? (a) N (c) S (b) P (d) H 13c. In the acid-base reaction that occurred in Wittig's experiment, the acid was: (a) (c) , CH3 HC- -N -CH3 H,C-N- -CH CH3 CH, (b) (d) CHA Li :CH 13d. Which one of these statements would be incorrect about tetramethylammonium ion? (a) The nitrogen is sp-hybridized. (b) The shape of the ion is tetrahedral. Name Date (c) The ion is polar. (d) Nitrogen has an octet of valence shell electrons
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


