Question: Complete the table. Temperature Trial 1 Trial 2 Trial 3 Concentration of iodide solution (mM) 100.0 100.0 100.0 Concentration of thiosulfate solution (mM) 20.1 20.1
Complete the table.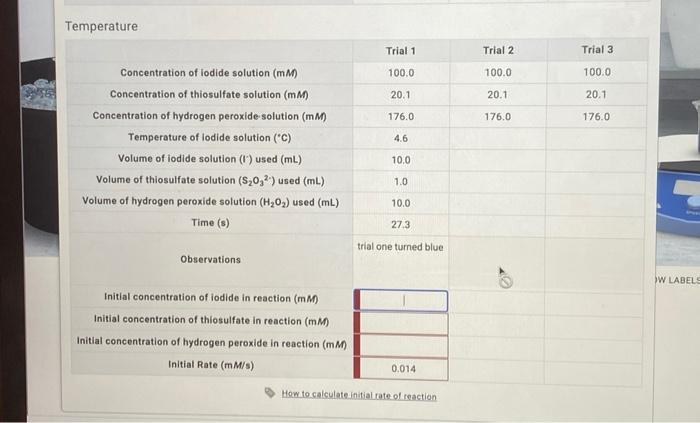
Temperature Trial 1 Trial 2 Trial 3 Concentration of iodide solution (mM) 100.0 100.0 100.0 Concentration of thiosulfate solution (mM) 20.1 20.1 20.1 Concentration of hydrogen peroxide solution (mM) 176.0 176.0 176.0 Temperature of lodide solution ("C) 4.6 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (S20,2) used (mL) 1.0 Volume of hydrogen peroxide solution (H202) used (mL) 10.0 Time (s) 27.3 trial one turned blue Observations W LABELS Initial concentration of iodide in reaction (mM) Initial concentration of thiosulfate in reaction (mM) Initial concentration of hydrogen peroxide in reaction (mM) Initial Rate (mM/s) 0.014 How to calculate.initial rate of.reaction
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


