Question: help me please A first-order decomposition reaction has a rate constant, k=0.0433min1. What is the half-life of this decomposition reaction? begin{tabular}{|} 10min hline 16min
help me please
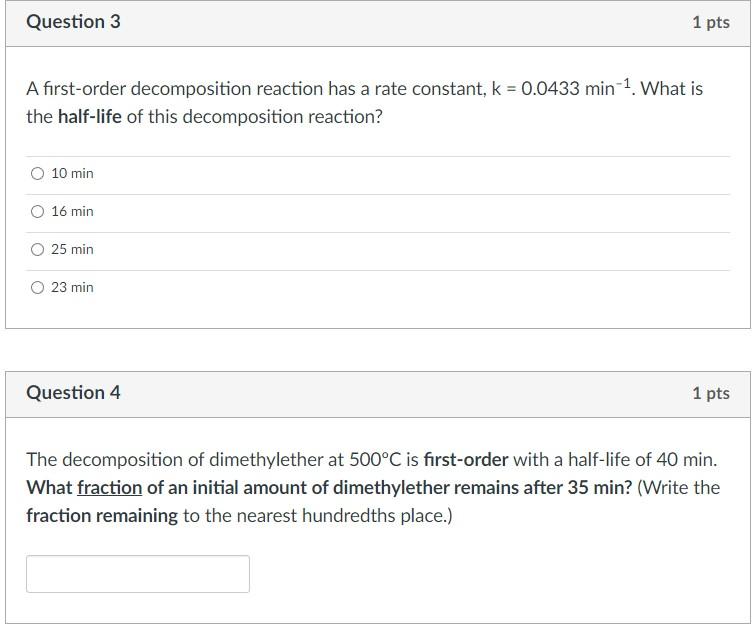
A first-order decomposition reaction has a rate constant, k=0.0433min1. What is the half-life of this decomposition reaction? \begin{tabular}{|} 10min \\ \hline 16min \\ \hline 25min \\ \hline 23min \end{tabular} Question 4 1pts The decomposition of dimethylether at 500C is first-order with a half-life of 40min. What fraction of an initial amount of dimethylether remains after 35min ? (Write the fraction remaining to the nearest hundredths place.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


