Question: help me please! Sample Problem No. 1 An old stock of a reagent bottle contains the label primary standard grade potassium hydrogen phthalate, KHCH.O, or
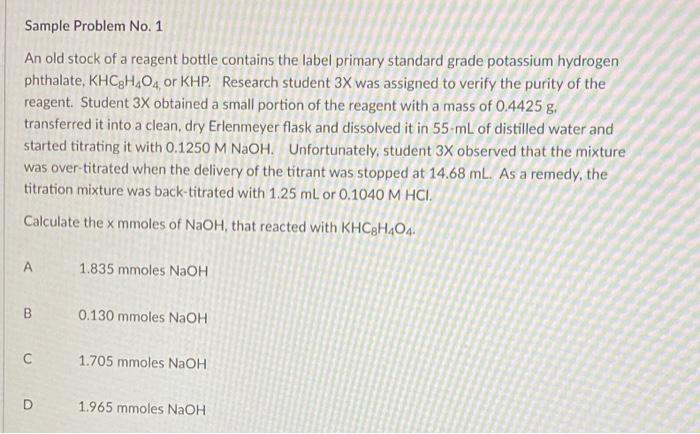
Sample Problem No. 1 An old stock of a reagent bottle contains the label primary standard grade potassium hydrogen phthalate, KHCH.O, or KHP. Research student 3X was assigned to verify the purity of the reagent. Student 3X obtained a small portion of the reagent with a mass of 0.4425 g. transferred it into a clean, dry Erlenmeyer flask and dissolved it in 55 mL of distilled water and started titrating it with 0.1250 M NaOH. Unfortunately, student 3X observed that the mixture was over-titrated when the delivery of the titrant was stopped at 14.68 ml. As a remedy, the titration mixture was back-titrated with 1.25 mL or 0.1040 M HCI. Calculate the x mmoles of NaOH, that reacted with KHC8H404. A 1.835 mmoles NaOH B 0.130 mmoles NaOH 1.705 mmoles NaOH D 1.965 mmoles NaOH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


