Question: help me to solve this question please quickly 1. A first-order reaction is 25% complete after 45 minutes. (a) Determine the rate constant, k. (5
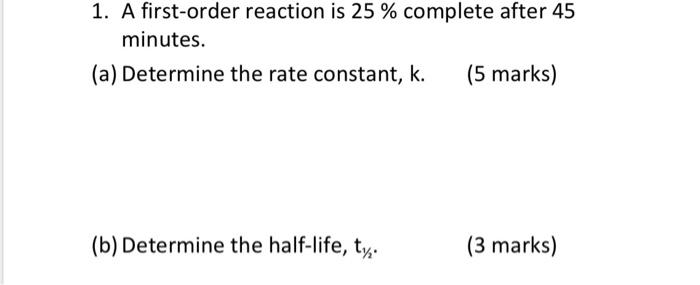

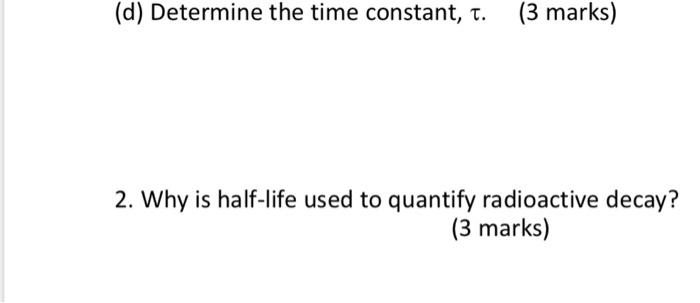
1. A first-order reaction is 25% complete after 45 minutes. (a) Determine the rate constant, k. (5 marks) (b) Determine the half-life, t1/2. (3 marks) (c) Derive the equations you have used in (a) and (b). (14 marks) (d) Determine the time constant, . (3 marks) 2. Why is half-life used to quantify radioactive decay
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


