Question: help on all but specifically answer B and explain and compare answer to lit values The vapor pressure of ethylene glycol at several temperatures is
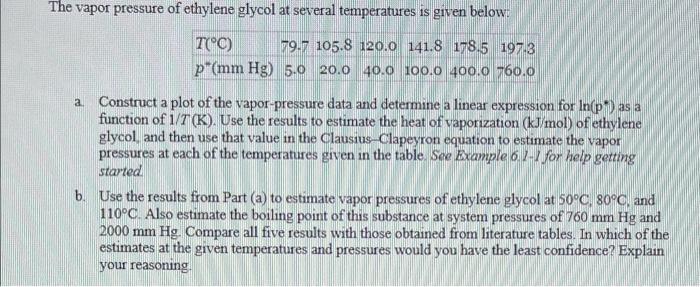
The vapor pressure of ethylene glycol at several temperatures is given below: a. Construct a plot of the vapor-pressure data and determine a linear expression for ln(p) as a function of 1/T(K). Use the results to estimate the heat of vaporization (kJ/mol) of ethylene glycol, and then use that value in the Clausius-Clapeyron equation to estimate the vapor pressures at each of the temperatures given in the table. See Example 611 for help getting started b. Use the results from Part (a) to estimate vapor pressures of ethylene glycol at 50C,80C, and 110C. Also estimate the boiling point of this substance at system pressures of 760mmHg and 2000mmHg. Compare all five results with those obtained from literature tables. In which of the estimates at the grven temperatures and pressures would you have the least confidence? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


