Question: Help on this please, I cant figure it out :( 3. Ethyl acetate is commonly used as a nail polish remover. For the process of
Help on this please, I cant figure it out :(
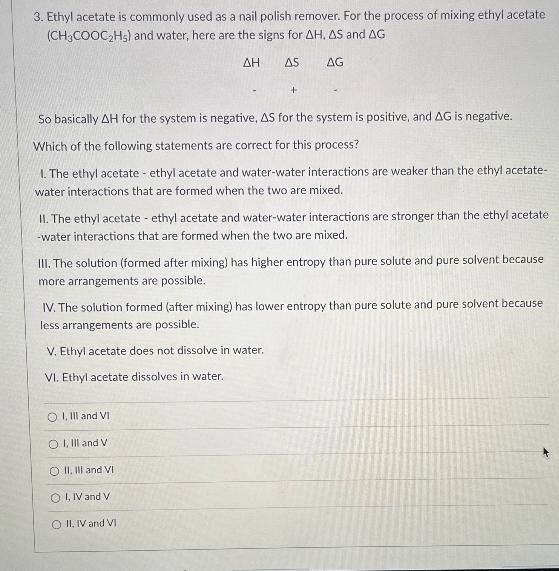
3. Ethyl acetate is commonly used as a nail polish remover. For the process of mixing ethyl acetate (CH3COOC2Hs) and water, here are the signs for AH. AS and AG AH AS AG + - So basically AH for the system is negative, AS for the system is positive, and AG is negative. Which of the following statements are correct for this process? 1. The ethyl acetate - ethyl acetate and water-water interactions are weaker than the ethyl acetate- water interactions that are formed when the two are mixed. I. The ethyl acetate - ethyl acetate and water-water interactions are stronger than the ethyl acetate -water interactions that are formed when the two are mixed. III. The solution (formed after mixing) has higher entropy than pure solute and pure solvent because more arrangements are possible. IV. The solution formed (after mixing) has lower entropy than pure solute and pure solvent because less arrangements are possible. V. Ethyl acetate does not dissolve in water. VI. Ethyl acetate dissolves in water. Ollll and VI O I, lll and V O II, III and VI O IIV and V II, IV and VI
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


