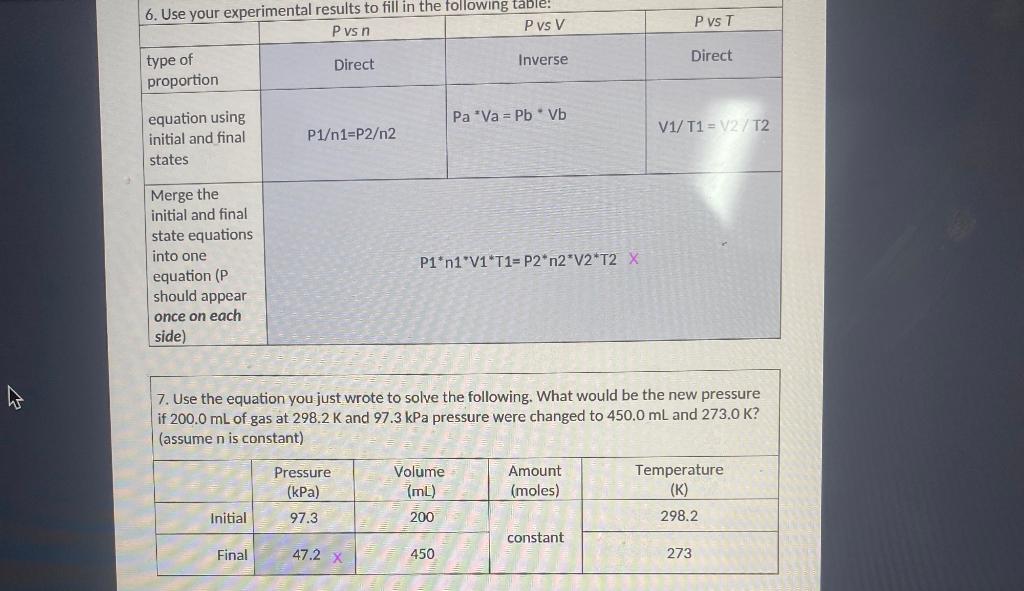
Question: help please 7. Use the equation you just wrote to solve the following. What would be the new pressure if 200.0mL of gas at 298.2K
 help please
help please
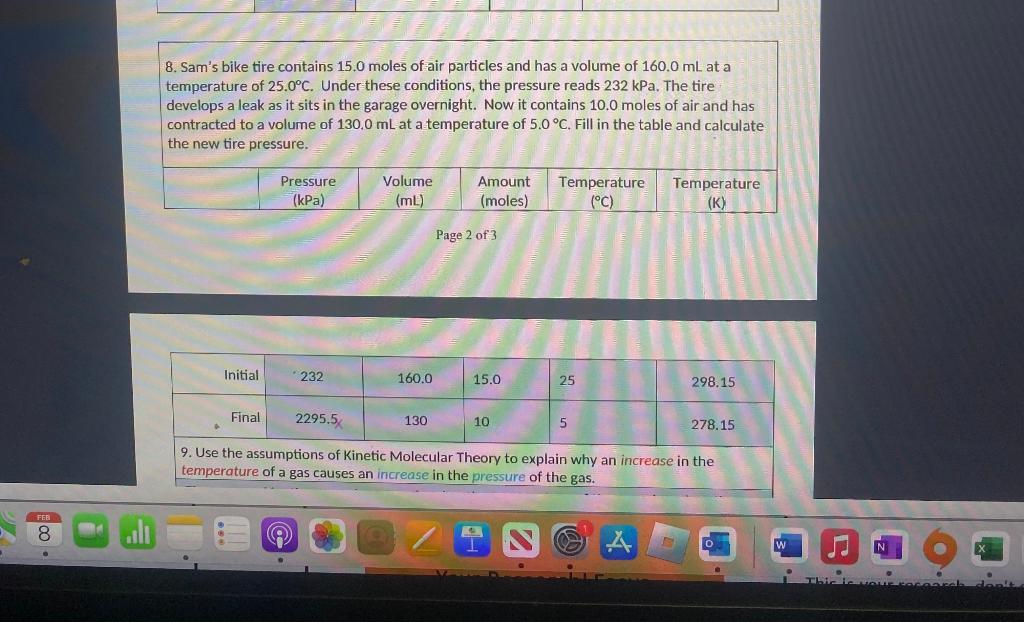
7. Use the equation you just wrote to solve the following. What would be the new pressure if 200.0mL of gas at 298.2K and 97.3kPa pressure were changed to 450.0mL and 273.0K? (assume n is constant) 8. Sam's bike tire contains 15.0 moles of air particles and has a volume of 160.0mL at a temperature of 25.0C. Under these conditions, the pressure reads 232kPa. The tire develops a leak as it sits in the garage overnight. Now it contains 10.0 moles of air and has contracted to a volume of 130.0mL at a temperature of 5.0C. Fill in the table and calculate the new tire pressure. Page 2 of 3 4. Use the assumptions of Kinetic Molecular Theory to explain why an increase in the temperature of a gas causes an increase in the pressure of the gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


