Question: help please. chart below Part C - Nat Ionic Earutlons (Use the data given in table on page 2) 1. Two possible net tonic equations

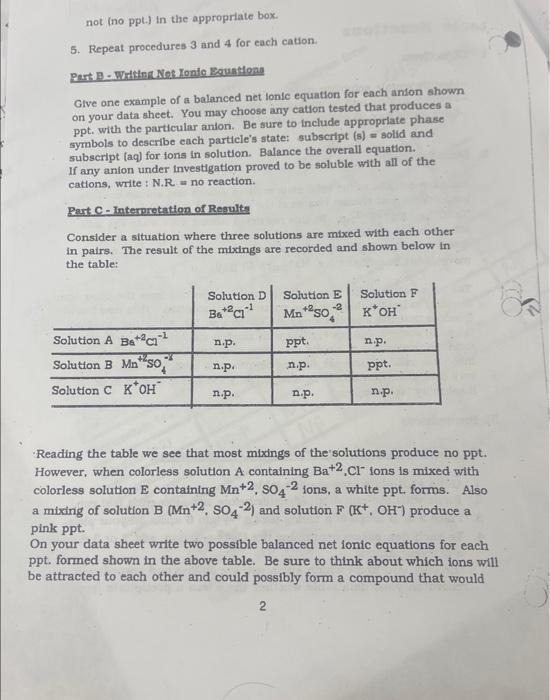
Part C - Nat Ionic Earutlons (Use the data given in table on page 2) 1. Two possible net tonic equations for the white pereipitate. Explanatlon: 2. Two possible net ionic equations for the pinik percipitate. Explanation: 5. Repeat procedures 3 and 4 for each cation. Pert. B - Whttent Net Ionic Eoustlons Give one example of a balanced net lonic equation for each anion shown on your data sheet. You may choose any cation tested that produces a ppt. with the particular anion. Be sure to include approprlate phase symbols to describe each particle's state: subseript (s)= solid and subscript (aq) for tons in solution. Balance the overall equation. If any anion under investigation proved to be soluble with all of the cations, write : N.R = no reaction. Part C - Interpretation of Regults Consider a situation where three solutions are mixed with each other in pairs. The result of the mixings are recorded and shown below in the table: Reading the table we see that most mixings of the solutions produce no ppt. However, when colorless solution A containing Ba+2,Cllons is mixed with colorless solution E containing Mn+2,SO42 ions, a white ppt. forms. Also a mixing of solution B(Mn+2,SO42) and solution F(K+,OH)produce a pink ppt. On your data sheet write two possible balanced net ionic equations for each ppt. formed shown in the above table. Be sure to think about which ions will be attracted to each other and could possibly form a compound that would
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


