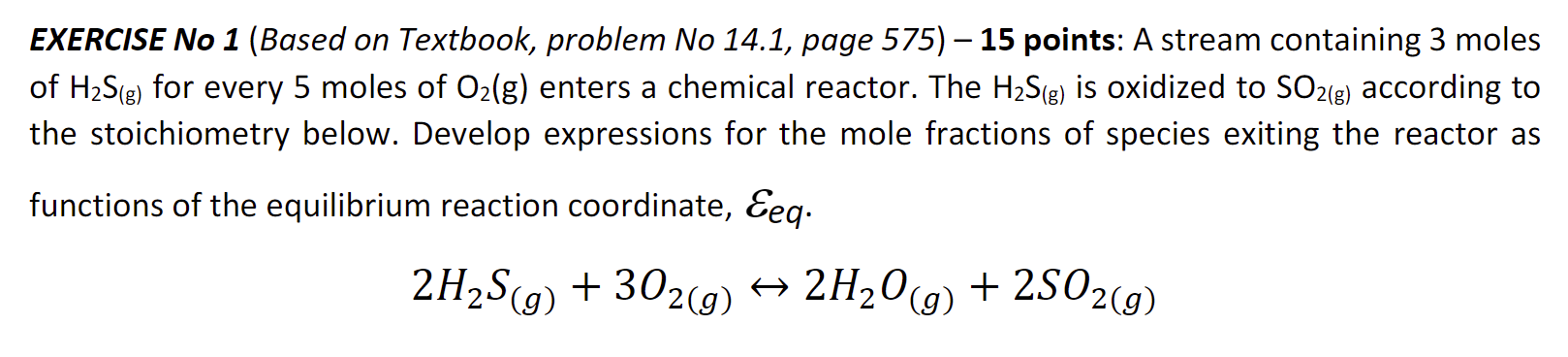
Question: Help please EXERCISE No 1 ( Based on Textbook, problem No 1 4 . 1 , page 5 7 5 ) - 1 5 points:
Help please EXERCISE No Based on Textbook, problem No page points: A stream containing moles
of for every moles of enters a chemical reactor. The is oxidized to according to
the stoichiometry below. Develop expressions for the mole fractions of species exiting the reactor as
functions of the equilibrium reaction coordinate,
harr
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


