Question: help please How many cm3 are there in 1m3 ? 1105cm31106cm3100cm31000cm3 Question 2 (1 point) Complete and balance the following equation. What are the products

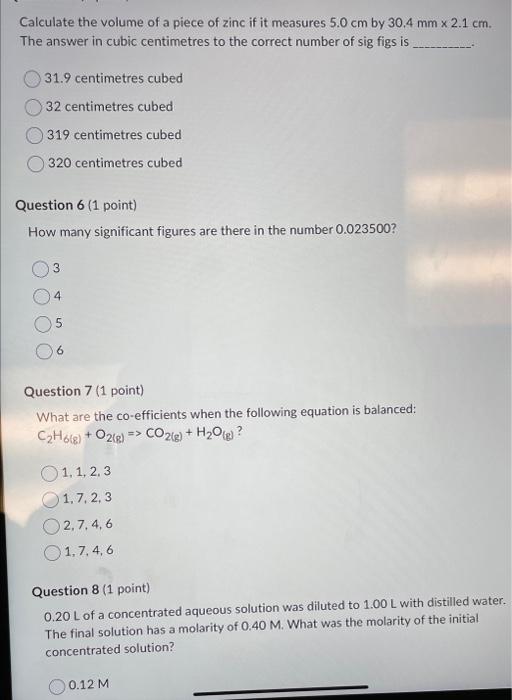
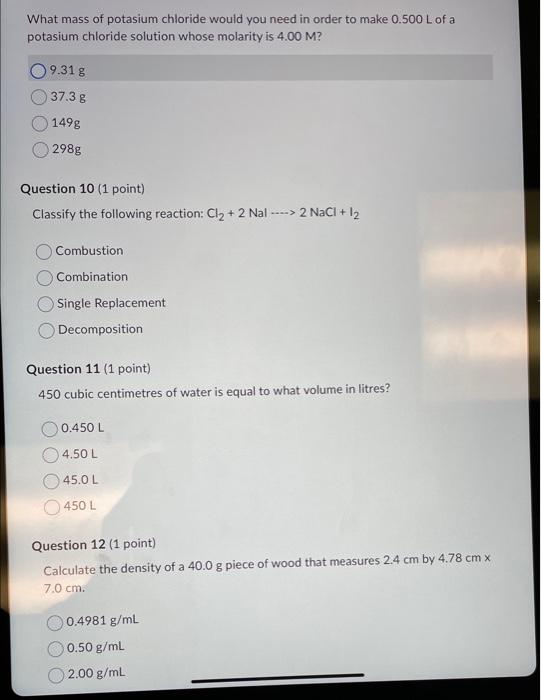

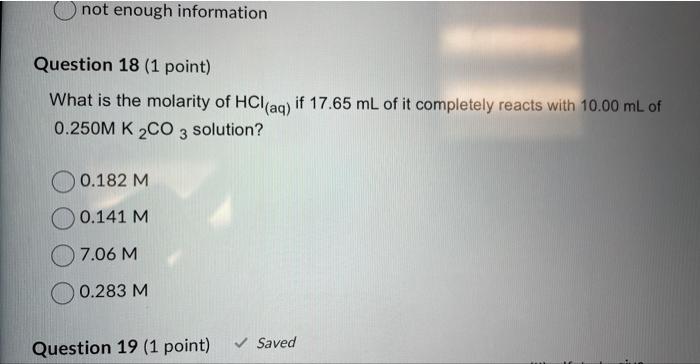
How many cm3 are there in 1m3 ? 1105cm31106cm3100cm31000cm3 Question 2 (1 point) Complete and balance the following equation. What are the products of the reaction ?CuSO4+NH4NO3 Cu(NO3)2+(NH4)2SO42CuNO3+(NH4)2SO4CuNO3+NH4SO4 no reaction occurs Question 3 ( 1 point) Calculate the mass in grams of 35.0mL of ether, which has a density of 0.708g/mL. 24.78g 24.8g 49.4g 49.43g Question 4 (1 point) Which of the following is the correct formula for iron (II) phosphate? Fe3(PO4)2Fe2PO4Fe2(PO4)3Fe(PO4)2 Calculate the volume of a piece of zinc if it measures 5.0cm by 30.4mm2.1cm. The answer in cubic centimetres to the correct number of sig figs is 31.9 centimetres cubed 32 centimetres cubed 319 centimetres cubed 320 centimetres cubed Question 6 ( 1 point) How many significant figures are there in the number 0.023500 ? Question 7 (1 point) What are the co-efficients when the following equation is balanced: C2H6(g)+O2(g)CO2(g)+H2O(g)? 1,1,2,3 1,7,2,3 2,7,4,6 1,7,4,6 Question 8 (1 point) 0.20L of a concentrated aqueous solution was diluted to 1.00L with distilled water. The final solution has a molarity of 0.40M. What was the molarity of the initial concentrated solution? 0.12M What mass of potasium chloride would you need in order to make 0.500L of a potasium chloride solution whose molarity is 4.00M ? 9.31g 37.3g 149g 298g Question 10 (1 point) Classify the following reaction: Cl2+2Nal>NaCl+I2 Combustion Combination Single Replacement Decomposition Question 11 (1 point) 450 cubic centimetres of water is equal to what volume in litres? \begin{tabular}{|} \hline 0.450L \\ 4.50L \\ 45.0L \\ 450L \\ \hline \end{tabular} Question 12 (1 point) Calculate the density of a 40.0g piece of wood that measures 2.4cm by 4.78cmx 7.0cm. 0.4981g/mL 0.50g/mL 2.00g/mL Aluminum oxide are produced according to the reaction given below: Reaction: 4Al+3O22Al2O3 If you start with 10.0g of Al and 19.0grams of O2 and the reaction goes to completion (all of the alumunium reacts) how much oxygen gas is left over? (Hint: Work from the theoretical yield from the last question to the amount of oxygen you would need and then subtract that from your original amount.) 31.5 gO2 remain unreacted 8.89gO2 remain unreacted 9.0gO2 remain unreacted 10.1 gO2 remain unreacted What is the molarity of HCl(aq) if 17.65mL of it completely reacts with 10.00mL of 0.250MK2CO3 solution? 0.182M 0.141M 7.06M 0.283M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


