Question: Help please!!! Need help with the bottom caulculations , I provided formulas and data ! please help D DATA 30.765 Mass of empty calorimeter (g)


Help please!!! Need help with the bottom caulculations , I provided formulas and data ! please help
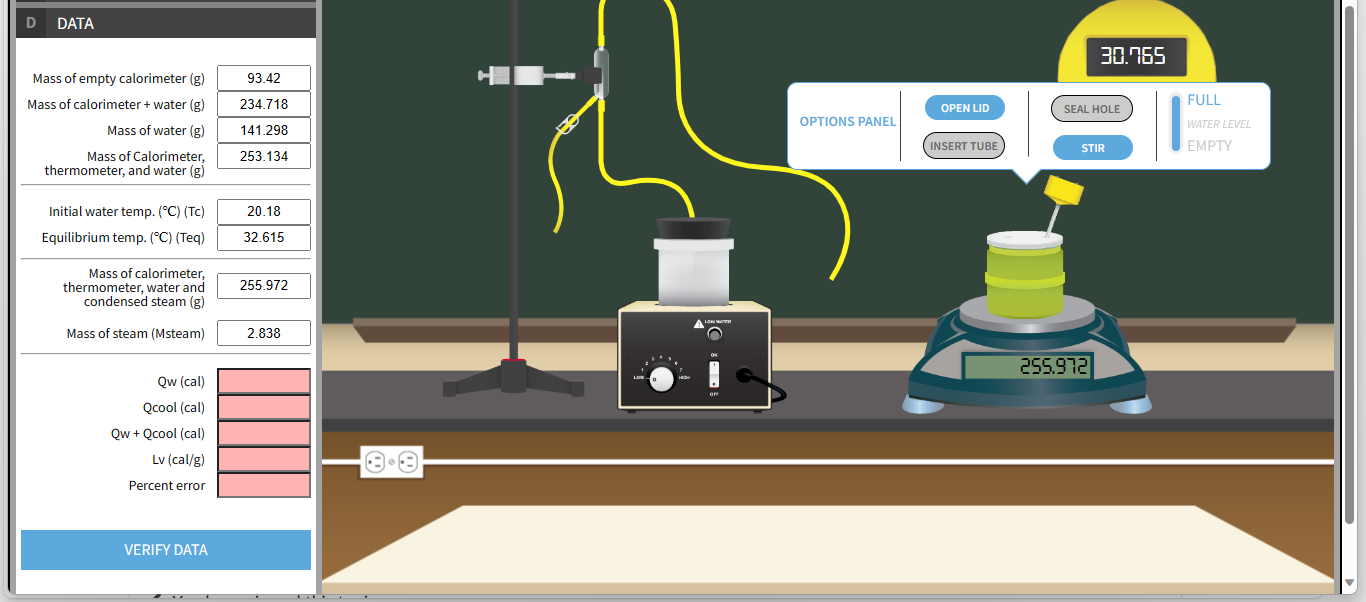


D DATA 30.765 Mass of empty calorimeter (g) 93.42 234.718 FULL Mass of calorimeter + water (g) OPEN LID SEAL HOLE OPTIONS PANEL WATER LEVEL Mass of water (g) 141.298 INSERT TUBE STIR EMPTY Mass of Calorimeter, 253.134 thermometer, and water (g) Initial water temp. ("C) (Tc) 20.18 Equilibrium temp. ("C) (Teq) 32.615 Mass of calorimeter, thermometer, water and 255.972 condensed steam (g) Mass of steam (Msteam) 2.838 ON 255.972 Qw (cal) Qcool (cal) Qw + Qcool (cal) Lv (cal/g) Percent error VERIFY DATAD DATA EXIT Mass of empty calorimeter (g In this experiment heat exchanges between two samples, steam and liquid water will be induced within Mass of calorimeter + water (g FULL a sealed container (a calorimeter) and the energy conservation principle will be used to determine the Mass of water (g WATER LEVEL latent heat of vaporization. The steam will emerge from a steam generator at approximately 100 C and EMPTY Mass of Calorimete thermometer, and water ( mix with the colder water in the calorimeter. The steam will lose energy as heat flows from the steam into the liquid water, and condenses forming 100 C water which will then cool, change temperature, in Initial water temp. (*C) (Tc contact with the colder water. The cold water will absorb heat energy, Qw through contact with the Equilibrium temp. (*C) (Teq steam. Mass of calorimete thermometer, water and Assuming the system remains isolated, energy conservation implies Qcond + Qcool + Qw = 0 condensed steam (g Mass of steam (Msteam equation 1) Qcond = -LvMsteam; energy loss through heat flows as steam condenses equation 2) Qcool = MsteamCw (Teq - 100.C); heat energy released as condensed steam cools in contact Qw (cal with cold water Qcool (cal equation 3) Qw = MwCw (Teq - Tc); heat energy absorbed by the cold water through contact with the condensed steam Qw + Qcool (cal Lv (cal/g Lv = Heat of vaporization of water Msteam = Mass of the steam Percent erro Cw = Specific heat of water 1.0 cal/gram 'c Tc = Initial temperature of cold water Mw = Mass of the cold water Teq = Equilibrium temperature of the mixture VERIFY DATD DATA EXIT Mass of empty calorimeter (g With Qw representing the heat energy absorbed by the water and Qcool representing the heat energy Mass of calorimeter + water (g FULL released as the hot water cools. By the conservation of energy the negative of this sum Qw + Qcool, is Mass of water (g WATER LEVEL equal to Qcond or EMPTY Mass of Calorimeter thermometer, and water (g (Qw + Qcool) = Qcond Initial water temp. ("C) (Tc We can then say... Equilibrium temp. (*C) (Teq (Qw+ Qcool) = -LvMsteam Mass of calorimeter thermometer, water and condensed steam (g And last, we convert the equation to... Mass of steam (Msteam Qw + Qcool equation 4) Lv = Msteam Qw (ca Qcool (cal The accepted value of the heat of vaporization of water is 539 cal/gram. Qw + Qcool (cal absolute value (experimental value of the heat of vaporization of water - accepted value) Lv (cal/g % error = X 100 accepted value of the heat of vaporization of water Percent errol VERIFY DAT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


