Question: help please Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis structure fails to describe accurately the
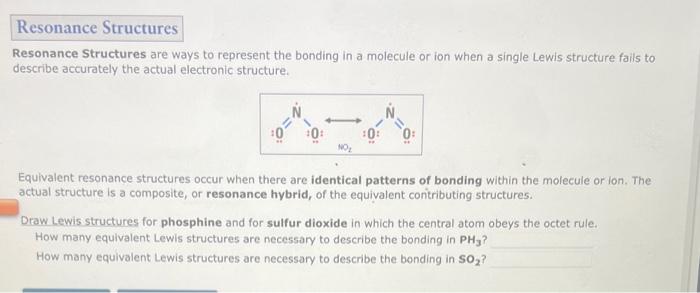
Resonance Structures are ways to represent the bonding in a molecule or ion when a single Lewis structure fails to describe accurately the actual electronic structure. Equivalent resonance structures occur when there are identical patterns of bonding within the molecule or ion. The actual structure is a composite, or resonance hybrid, of the equivalent contributing structures. Draw Lewis structures for phosphine and for sulfur dioxide in which the central atom obeys the octet rule. How many equivalent Lewis structures are necessary to describe the bonding in PH3 ? How many equivalent Lewis structures are necessary to describe the bonding in SO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


