Question: help plz 5) (5 pts) A first order reaction ( A2B) has a half-life of 5.40 minutes. If a reaction starts with [A]0=0.156M, what is
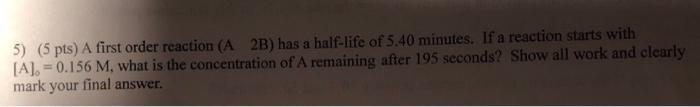
![a half-life of 5.40 minutes. If a reaction starts with [A]0=0.156M, what](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f90e3a56d24_13866f90e3a0ae48.jpg)
5) (5 pts) A first order reaction ( A2B) has a half-life of 5.40 minutes. If a reaction starts with [A]0=0.156M, what is the concentration of A remaining after 195 seconds? Show all work and clearly mark your final answer. 5) (5 pts) A first order reaction ( A2B) has a half-life of 5.40 minutes. If a reaction starts with [A]0=0.156M, what is the concentration of A remaining after 195 seconds? Show all work and clearly. mark your final
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


