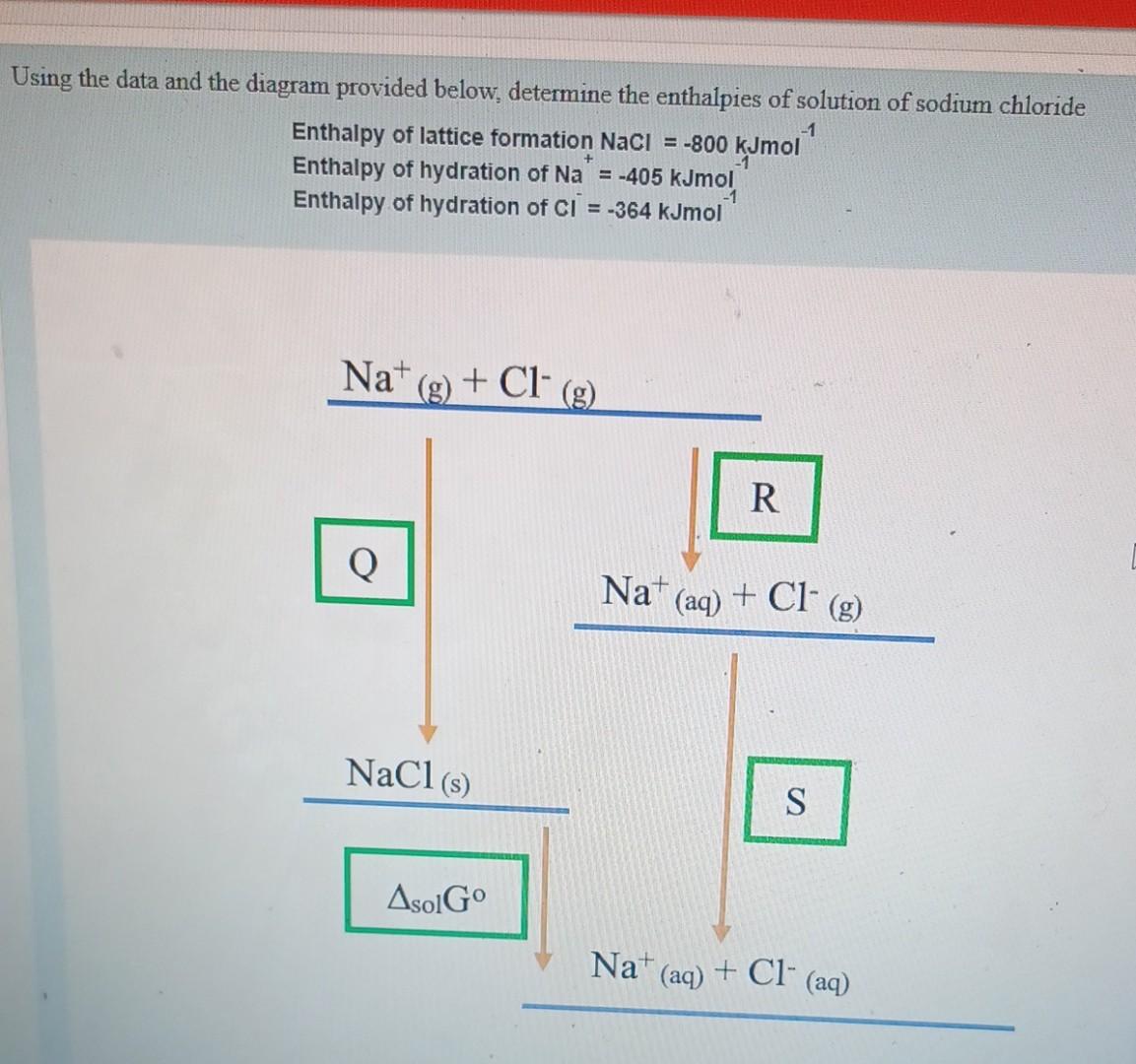
Question: help plz Using the data and the diagram provided below, determine the enthalpies of solution of sodium chloride Enthalpy of lattice formation NaCl=800kJmol1 Enthalpy of
help plz
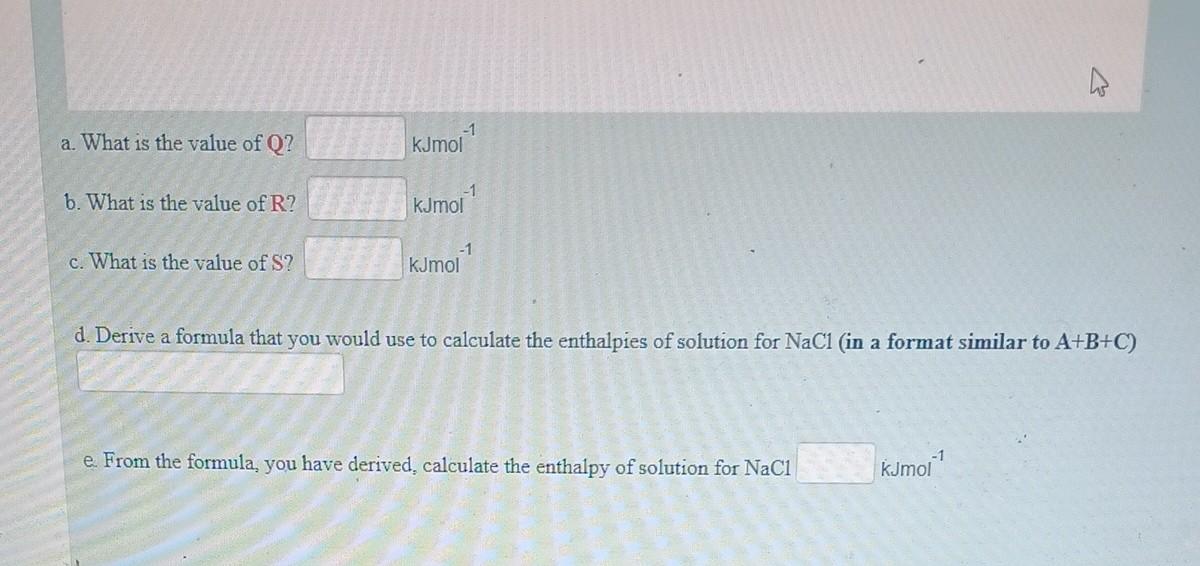
Using the data and the diagram provided below, determine the enthalpies of solution of sodium chloride Enthalpy of lattice formation NaCl=800kJmol1 Enthalpy of hydration of Na+=405kJmol1 Enthalpy of hydration of Cl=364kJmol1 a. What is the value of Q ? kJmol1 b. What is the value of R ? kJmol1 c. What is the value of S ? kJmol1 d. Derive a formula that you would use to calculate the enthalpies of solution for NaCl (in a format similar to A+B+C ) From the formula, you have derived, calculate the enthalpy of solution for NaCl kJmol 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


