Question: please answer Using the data and the diagram provided below, determine the enthalpies of solution of silver chloride Enthaipy of lattice formation AgCl=905kJmol1 Enthatpy of
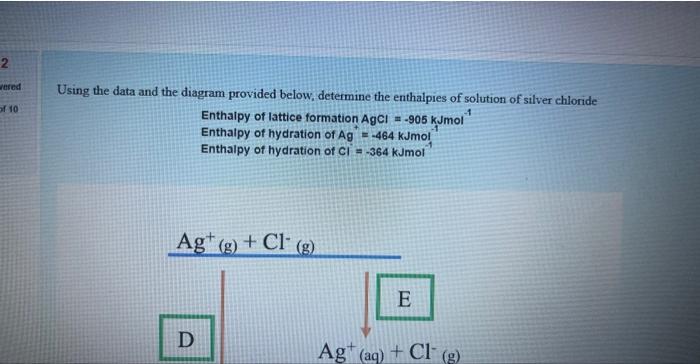
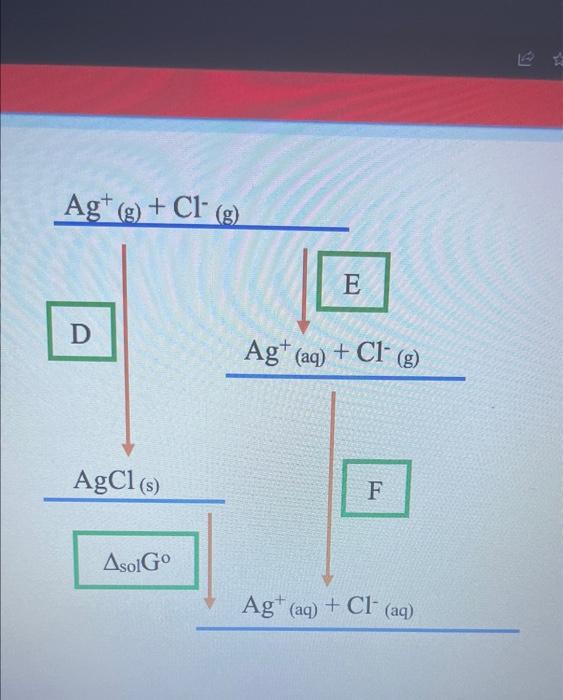
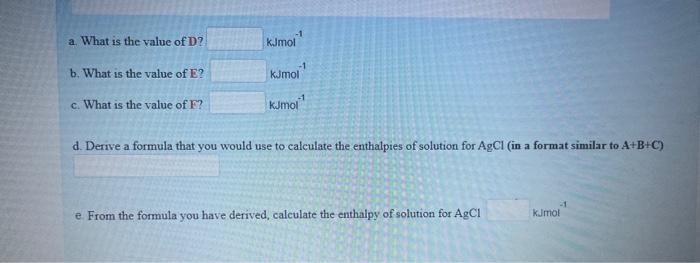
Using the data and the diagram provided below, determine the enthalpies of solution of silver chloride Enthaipy of lattice formation AgCl=905kJmol1 Enthatpy of hydration of Ag+=464kJmol1 Enthalpy of hydration of Cl=364kJmol1 a. What is the value of D? b. What is the value of E ? kjmol1 c. What is the value of F ? kJmol1 d. Derive a formula that you would use to calculate the enthalpies of solution for AgCl (in a format similar to A+B+C ) From the formula you have derived, calculate the enthalpy of solution for AgCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


