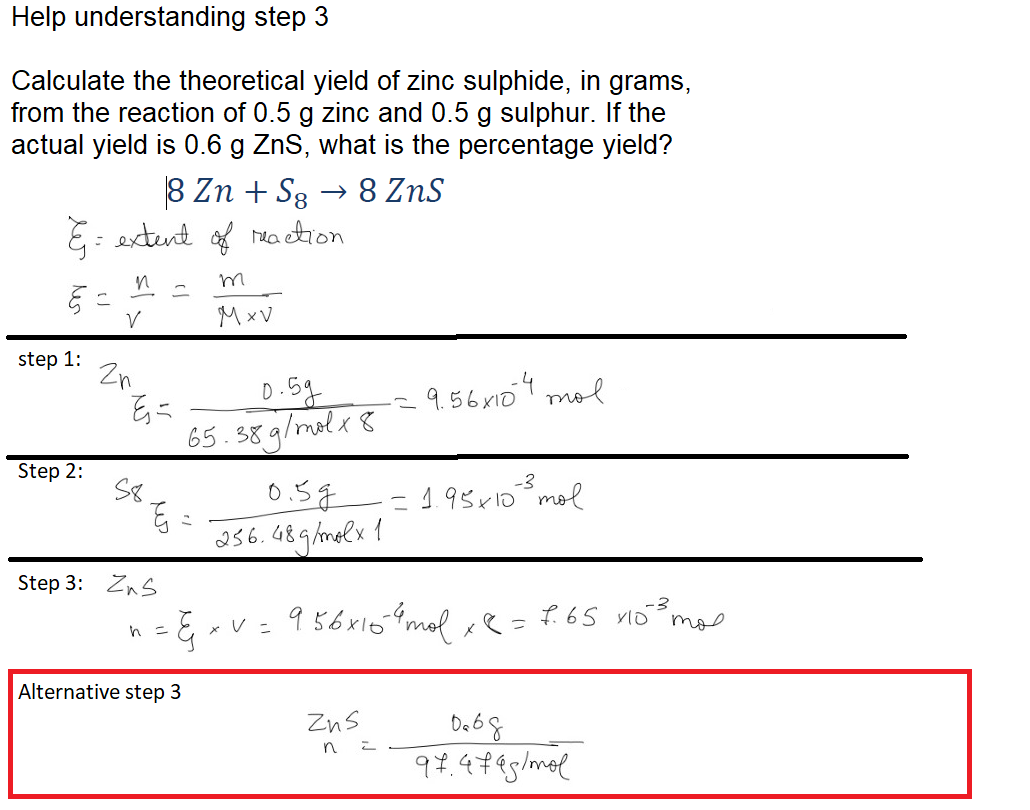
Question: Help understanding step 3 . Can someone explain why I can't use Alternative step 3 instead of 3 since we have mass of ZnS produced.
Help understanding step Can someone explain why I can't use Alternative step instead of since we have mass of ZnS produced. And why step uses Zns exten of reaction?
Question: Calculate the theoretical yield of zinc sulphide, in grams, from the reaction of zinc and sulphur. If the actual yield is what is the percentage yield?
extent of raction
step :
mol
Step :
mol
Step :
molmol
Alternative step
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


