Question: help!! When a 6.00 g sample of KBr is dissolved in water in a calorimeter that has a total heat capacity of 2.87X K-the temperature
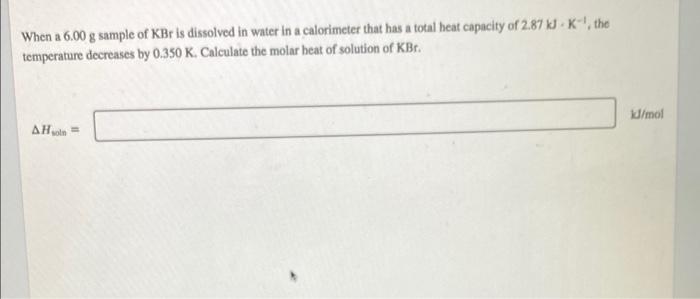
When a 6.00 g sample of KBr is dissolved in water in a calorimeter that has a total heat capacity of 2.87X K-the temperature decreases by 0.350 K. Calculate the molar heat of solution of KBr. /mol A Hon =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


