Question: please write neatly if you can and avoid typing please thank you 8-49. A 5.00-g sample of potassium chloride is dissolved in 1.00 L of


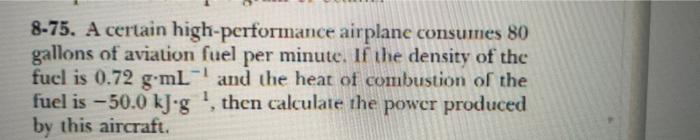
8-49. A 5.00-g sample of potassium chloride is dissolved in 1.00 L of water in a calorimeter whose heat capacity is 4.51 kJ-K- The temperature decreases 0.256 K. Calcu- late the molar beat of solution of potassium chloride. 8-67. The mineral stilleite contains zinc and selenium. The observed heat capacity per gram is 0.348 J-K 'g Use Dulong and Petit's rulc (Problem 8-65) to determine the forinula of sulleite. 8-75. A certain high-performance airplane consumes 80 gallons of aviation fuel per minute. If the density of the fuel is 0.72 g ml and the heat of combustion of the fuel is - 50.0 kJog, then calculate the power produced by this aircraft
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


